EPIDEMIOLOGY
About 4% to 15% patients referred for uveitis have pars planitis.10–17 Pars planitis usually affects children and young adults.18 It seldom develops before age 5 or after age 30. Most cases develop in the teens and early twenties. In most cases pars planitis enters remission before age 40. Some reports10,18 show no racial predilection, but others11,13,16 show that whites are affected more frequently than blacks. There is no predilection toward either sex.10,18 Pars planitis is bilateral about 71% to 75% of the time.4,13,19 Familial cases of pars planitis and intermediate uveitis have been reported.20–24
SYMPTOMS
The onset of symptoms is very gradual in pars planitis. The most common symptoms of pars planitis are blurred vision and floaters. Symptoms may be present for months to years before the patient seeks medical attention. At times, patients with pars planitis are asymptomatic, and the diagnosis is made on a routine ophthalmic examination. Patients with pars planitis rarely report redness, pain, or photophobia.
SIGNS
Externally, the eyes of patients with pars planitis appear uninflamed. The conjunctiva and sclera are usually white without injection. The cornea is usually clear and appears uninvolved. In long-standing cases, band keratopathy may develop, most frequently in patients who developed pars planitis in childhood or their early teens.11,13 In some cases of pars planitis, small keratic precipitates and fibrin may be present on the corneal endothelium. Large keratic precipitates are not characteristic of pars planitis. The anterior chamber may be clear or show only mild cells and flare. The amount of inflammation in the anterior chamber is typically mild, rarely exceeding grade 2+ .25 The iris may be uninvolved or show only one or two localized posterior synechiae. Extensive iris synechiae, seclusion of pupil, and iris nodules are not typically seen in pars planitis. The lens may be clear or may appear cataractous. In pars planitis, posterior subcapsular cataract is most frequently seen, and it may be the result of ocular inflammation or the chronic use of corticosteroids.
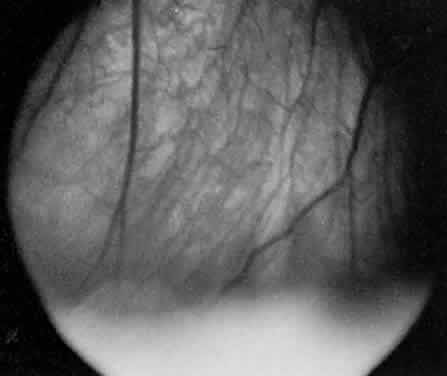
In pars planitis, the inflammatory signs are greatest in the vitreous cavity. The vitreous gel is syneretic and shows varying amount of fibrin, cells, and strands. The hallmark of pars planitis is the presence of exudates in the inferior vitreous base (Fig. 1). In early stages these exudates may appear as discontinuous yellow-white clumps (fluffballs or snowballs). However, as the disease progresses, these exudates may increase in number and size until they coalesce to form a fluffy white exudate over the inferior peripheral retina and pars plana. Later, the exudate organizes into a smooth white fibrous-appearing band. This membrane has been termed a snowbank because of its resemblance to white fluffy snow. The term pars planitis is reserved for intermediate uveitis in which snowbank is present. The amount of vitreous inflammation is usually symmetric in both eyes. However, in some cases, a prominent snowbank may be present in one eye but only a few fluff balls in the other. Scleral indentation is often required to visualize the snowbank (Fig. 2).7,26
|
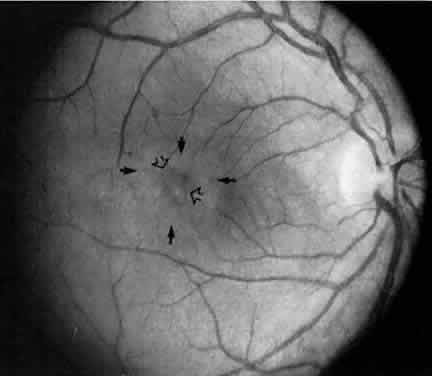
Retinal changes that can occur in pars planitis include perivascular sheathing of the retinal venules (periphlebitis), cystoid macular edema, and optic disc edema (Figs. 3 and 4). In cases of chronic cystoid macular edema, epiretinal membrane formation often occurs.4,13,27
|
|
FLUORESCEIN ANGIOGRAPHY AND VITREOUS FLUOROPHOTOMETRY
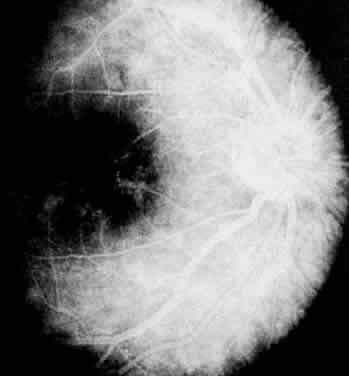
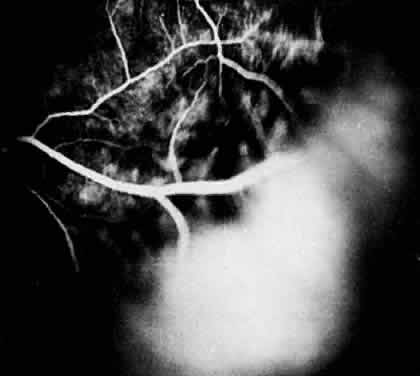
In pars planitis, fluorescein angiography shows diffuse leakage from the retinal venules and capillaries, without any predilection for the inferior retinal vasculature (Figs. 5 and 6).28 Similarly, vitreous fluorophotometry in patients with pars planitis also shows no predilection for vascular leakage in the inferior peripheral retina.29 These findings suggest that snowbank is most likely a sequela from ocular inflammation, that inferior peripheral retina periphery is not the source of inflammation in pars planitis, and that pars planitis is not a localized inflammatory response of the inferior retina.
|
|
CLINICAL COURSE
The clinical course is marked by chronic low-grade inflammation, which may be punctuated by episodes of exacerbation. In a study of 100 patients, Smith and colleagues13 divided the clinical course of their patients into three categories. The first group (10% of the eyes) followed a benign self-limiting course of gradual clinical improvement without episodes of exacerbation. The second group (59% of the eyes) followed a smoldering, prolonged course without episodes of exacerbation. The third group (31% of the eyes) followed a smoldering and prolonged course with episodes of exacerbation. About 4% of their cases underwent spontaneous remission.
In pars planitis, the severity of the disease has no correlation to the duration of disease. Cases that are unilateral at first presentation may become bilateral later. Pars planitis is bilateral about 71% to 75% of the time.4,13,19 Often there is asymmetry in severity between eyes. In our experience, unilateral cases tend to have a milder course than bilateral cases. The presence of snowbank may indicate more severe and prolonged vitreous inflammation and often is associated with cystoid macular edema.30 In most of the patients, the clinical course becomes milder with time, and it usually enters remission between the patient's midthirties and early forties.
HISTOPATHOLOGY
Few pathologic reports of pars planitis have been described. Most cases are long-standing and chronic. Early changes have not been reported.
Histologically, the snowbank is composed of glial elements, type VI collagen, and laminin.31 Extensive fibroglial proliferation and fibrous astrocyticlike cells have been seen in the vitreous base.32,33 These cells can synthesize basement membranes and produce large-diameter collagen fibrils.32,33 Fibrovascular tissue may be present in the snowbank and consists of well-differentiated capillaries that probably originate from the peripheral retina.33
The vitreous is usually collapsed and detached posteriorly from the retina. Multinucleated giant cells and epithelioid cells have been demonstrated in the vitreous snowballs.34 There is cellular proliferation from the retina and hyperplasia of the nonpigmented ciliary epithelium.34 The choroid usually does not show significant inflammation.31,34
Lymphocytic cuffing and mural infiltration of the retinal venules can be present, consistent with periphlebitis and phlebitis, respectively.33 The retinal arterioles are spared.33 These lymphocytes are mostly T-helper cells.31 Histologic changes consistent with clinical cystoid macular edema may be present in the fovea.33
ETIOLOGY AND PATHOGENESIS
The cause of pars planitis is unknown, and its pathogenesis remains unclear. As described in previous sections, inflammation in pars planitis is most prominent in the vitreous and seems to begin clinically in the inferior vitreous base, with formation of fluff balls during the early stages of disease. This suggests that the pathogenesis of pars planitis begins in the vitreous base. Snowbanks, resembling those in pars planitis, have been produced in monkeys35 receiving multiple intravitreal injections of hyaluronic acid and in rabbits36 receiving intravitreal injections of crystalline egg albumin. Further, a predominance of T-helper cells has been demonstrated in the snowbank glial tissue in one eye.31 These findings suggest that deposition of foreign antigens in the vitreous may lead to pars planitis.
The predilection for the inferior vitreous base to form snowbank is perhaps due to the gravitational settling of cells and debris from the inflamed vitreous in pars planitis. However, the formation of snowbank may also be due to an increased number of cells in the inferior vitreous.37
Elevated serum levels of IgD have been reported in patients with pars planitis.38 Humoral and cell-mediated immunity against the photoreceptor S-antigen have been demonstrated in some patients with pars planitis.39–41 The presence of retinal immunity may represent an epiphenomenon that occurs after the initial insult to the retina.39 A similar epiphenomenon has been observed in patients with diabetic retinopathy; they also show elevated serum levels of anti-S-antigen antibody after undergoing retinal photocoagulation.42 However, even if not primary, retinal autoimmunity may exacerbate the inflammation in pars planitis. Class II antigen has been demonstrated on the vascular endothelium in eyes with pars planitis, indicating that the vascular endothelium may also be part of the immunologic process.31
Electrophysiologic studies have shown B-wave abnormalities, suggesting a vitreoretinal disorder rather than a primary uveal disease in pars planitis.43
Linearly arranged keratic precipitates on the inferior corneal endothelium have been described in patients with pars planitis.44,45 Khoudadoust and coworkers44 proposed that these linear keratic precipitates are the results of autoimmune corneal endotheliopathy. However, Pivetti-Pezzi and Tamburi46 did not identify any cases of autoimmune corneal endotheliopathy in a retrospective study of 58 patients. It is doubtful that autoimmune corneal endotheliopathy and pars planitis are related disorders, and it is most likely that the keratic precipitates in pars planitis are from chronic anterior chamber reactions spilled over from the vitreous inflammation.47,48
Familial cases11,20–24,31,49 of pars planitis have been reported, suggesting a possible genetic predilection or a common environmental factor that may predispose certain individuals to develop pars planitis. Recently, human leukocyte antigen (HLA) DR15 has been associated with pars planitis.50,51 HLA-DR15 is also associated with MS, optic neuritis, and narcolepsy.50 HLA-DR15 may be a marker that predisposes some to develop pars planitis. Further, pars planitis may be a part of the disease spectrum associated with HLA-DR15.50 This is reminiscent of disorders related to HLA-B27, which include acute iridocyclitis, ankylosing spondylitis, Reiter's syndrome, ulcerative colitis, and psoriatic arthritis.
COMPLICATIONS
The most frequent complication in pars planitis is cystoid macular edema, which occurs in about 30% of cases.13,52 Cataracts have been reported in 5%52 to 42%13 of cases. With time, the percentage of pars planitis patients with visual loss due to macular disease tends to increase, whereas the percentage of pars planitis patients with visual loss due to cataracts and vitreous opacities tends to decrease.13 Cystoid macular edema and snowbank have been reported to be associated with a worse visual outcome.52
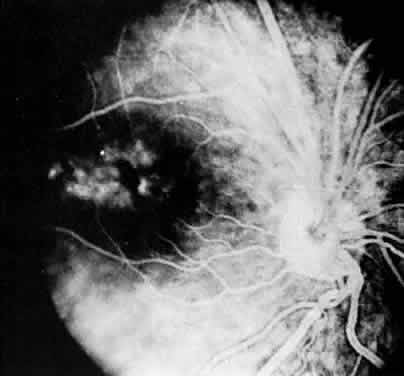
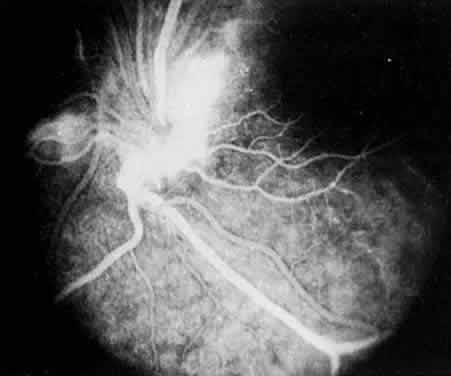
Retinal detachment, vitreous hemorrhage, optic disc swelling, and dragging of the optic disc vessels caused by contraction and neovascularization of cyclitic membranes occur in less than 10% of cases.13,52,53 Periphlebitis has been reported in about 21% of cases.52 Neovascularization of the optic disc53–55 and peripheral retina56 can also occur (Fig. 7). Coat's-like response has been reported in pars plantitis.57 Band keratopathy, glaucoma, and retinoschisis may develop as late sequelae in pars planitis.13
|
DIAGNOSIS
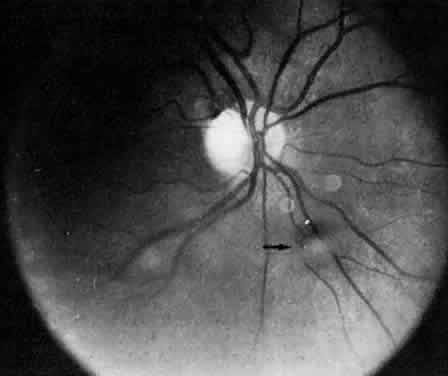
The diagnosis of pars planitis is based completely on history and clinical findings. Pars planitis is a chronic uveitis in which the most severely inflamed part of the eye is the vitreous. The vitreous should show the greatest concentration of inflammatory cells. Pars planitis is also characterized by the presence of vitreous fluff balls (Fig. 8) or a snowbank in the inferior vitreous base. Macular edema is another important feature in pars planitis, because pars planitis is one of the most common causes of cystoid macular edema in young adults. Pars planitis is a disease of the young and early middle-aged adults. The onset of pars planitis is usually in the late teens and early twenties; it rarely occurs after age 30.
|
The differential diagnosis includes chronic iridocyclitis and intermediate uveitis secondary to sarcoidosis. The relative paucity of anterior chamber cells and the presence of snowbank in pars planitis distinguish pars planitis from chronic iridocyclitis. Posterior synechiae tend to be larger and more numerous in chronic iridocyclitis than in pars planitis. However, children with pars planitis, younger than 12, frequently have an anterior chamber reaction with severe synechiae and band keratopathy, which are features more characteristic of chronic iridocyclitis than pars planitis. It is unclear whether these severe childhood cases represent true intermediate uveitis or severe chronic iridocyclitis with secondary cyclitic membrane formation.
Chronic iridocyclitis and intermediate uveitis secondary to sarcoidosis can resemble pars planitis. Patients with sarcoidosis tend to have more prominent sheathing of the retinal venules and larger mutton-fat-like keratic precipitates. Retinal, choroidal, and optic disc granulomas may form in patients with sarcoidosis. Opacities resembling snowballs or a string of pearls in the inferior peripheral vitreous are more characteristic of sarcoidosis than pars planitis.
Unilateral cases of pars planitis must be carefully evaluated for toxoplasmosis and toxocariasis.58 In both of these conditions, areas of inflammation are usually more focal than those in pars planitis. Old chorioretinal scars and vitreous cells emanating from focal areas of retinochoroiditis may be helpful in making the diagnosis of ocular toxoplasmosis. Peripheral retinal granuloma and dragging of the disc by fibrovascular tissue can occur in toxocariasis. A case of intermediate uveitis after cataract surgery has been found to be caused by chronic Propionibacterium acnes endophthalmitis.59
The laboratory evaluation of a patient with pars planitis includes a chest x-ray, analysis of serum lysozyme and angiotensin-converting enzyme levels, and serology for syphilis. When toxoplasmosis or toxocariasis is strongly suggested, appropriate laboratory tests should be ordered. In some patients, pars planitis and MS are present concomitantly. If MS is suspected, neurologic consultation is recommended, and checking for HLA-DR15 may be helpful. Pars planitis has also been reported in patients with Lyme disease.60,61 Infectious disease consultation and appropriate tests should be obtained, especially when there is a history of tick bite in endemic areas for Lyme disease. In the future, the polymerase chain reaction may be performed on aqueous humor or vitreous from eyes with pars planitis to identify possible infectious agents.
TREATMENT
The mainstay of treatment of pars planitis is corticosteroids. The corticosteroid can be administered topically, orally, periocularly, or intravenously. Topical corticosteroids, such as prednisolone acetate or phosphate, are generally useful in treating the anterior chamber reaction that may be present in some pars planitis patients. Topical corticosteroids, however, are of little use to control the vitreous inflammation or the cystoid macular edema. Topical corticosteroids are generally used as an adjunct in treatment of pars planitis when there is a significant anterior chamber reaction. Cycloplegia may also be necessary to prevent posterior syne-chiae formation. Patients taking topical corticosteroids should be followed regularly, because prolonged use of topical corticosteroids may lead to elevated intraocular pressure and cataract formation.
We generally recommend the periocular injection of steroids into the posterior sub-Tenon's space in cases of significant vitreous reaction, cystoid macular edema, or retinal periphlebitis. Posterior sub-Tenon's injection provides the highest concentration of corticosteroids to the most inflamed part of the eye in pars planitis. We generally define visual acuity of 20/40 or less due to vitreous cells and floaters as significant, but obviously significant visual loss must be defined individually. A young pars planitis patient may have a visual acuity of 20/25 with significant clinical cystoid macular edema, whereas an older patient may have a visual acuity of 20/40 with no clinically significant macular edema. The loss of vision in the older patient may be due to permanent macular changes from previous macular edema. We frequently and periodically use fluorescein angiography to monitor the extent of macular edema. If both cataract and cystoid macular edema are present, laser interferometry or potential acuity testing may be useful to assess potentialmacular function. However, these tests frequentlyare overly optimistic in their prediction of im-provement.
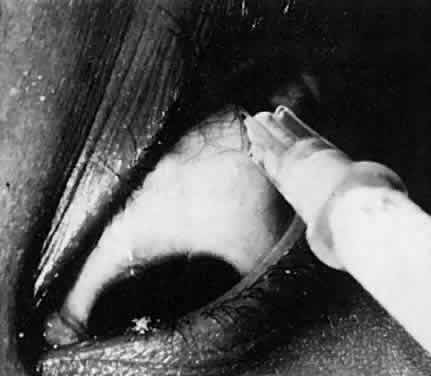
In giving posterior sub-Tenon's injection, we follow the technique of Nozik,62 in which the corticosteroid is placed adjacent to the sclera posterior to the equator through the superotemporal conjunctival fornix (Fig. 9). We use a 25-gauge needle that is 5/8 long and attached to a tuberculin or 3-mL syringe. Under topical anesthesia, the patient sits in a chair with the back of the head against the headrest. The patient is instructed to look down in the direction of the elbow contralateral to the eye that is to receive the injection. The upper eyelid is lifted with the nondominant hand, and the dominant hand is used to direct the needle. The superotem-poral conjunctival fornix is penetrated with the needle bevel up. After the tip of the needle enters the fornix, the needle is rotated 180 degrees with bevel down. The needle is advanced along the sclera posteriorly slowly with a side-to-side sweeping motion until reaching the hub of the needle, and the injection is then given. When given properly, no medication should be visible anterior to the equator. Careful attention is given to the globe during the advancement of the needle; the movement of the globe should be far less than the sweeping motion of the needle. If there is an exact correspondence between the movements of the globe and the needle, the tip of the needle may be engaging the sclera, and the needle should be withdrawn slightly and then again carefully advanced. The goal is to have the corticosteroid delivered adjacent to the posterior sclera without penetrating the globe.
|
Freeman and associates63 have shown by ultrasound that the superotemporal placement technique results in more accurate placement of steroids near the macula. They demonstrated that through the superotemporal approach, the gravitational effect on the injected volume would pull the medication closer to the posterior pole, compared with the inferotemporal approach. In the latter approach, gravity should pull the medication away from the globe. The injection can be repeated every 2 to 3 weeks. The dose can vary from 0.5 mL of triamcinolone diacetate (Aristocort; 40 mg/mL) to 1 mL of methylprednisolone acetate (Depo-Medrol; 80 mg/mL). Triamcinolone diacetate is more soluble than triamcinolone acetonide (Kenalog). When there is concern about a pressure elevation, the diacetate might preferably be used because it dissipates after about 3 months, whereas the acetonide can cause problems for over 1 year. Methylprednisolone acetate is more apt to cause allergic reactions and fibrosis than the triamcinolone preparations. Posterior sub-Tenon's injection is an especially effective therapy in the treatment of cystoid macular edema in pars planitis, and it is also useful in reducing the cellular activity in the vitreous.64,65 The success rate in improving visual acuity with posterior sub-Tenon's injection is about 80% to 90%.64,65 Posterior sub-Tenon's injection is also useful in reducing the cellular activity in the vitreous in about 75% of cases.65
Close attention must be given to the intraocular pressure, which may become elevated after periocular corticosteroid injection. It is worth using topical corticosteroids four times a day for 2 weeks to eliminate injections in patients who are exquisite steroid-glaucoma responders. Lafranco and coworkers64 reported an incidence of 36% of elevated intraocular pressure greater than 8 mmHg in their series of 58 eyes receiving a total of 162 posterior sub-Tenon's injections. From our experience, when the injection is given properly, elevated intraocular pressure is seldom encountered with posterior sub-Tenon's injection of corticosteroids. With periocular injections, other risks include ocular perforation, worsening cataract, and superior eyelid ptosis.64,65
We use oral corticosteroids when there is exacerbation of inflammation in both eyes, when the patient cannot receive periocular injections of corticosteroids, or when periocular injections fail to work. Sometimes oral corticosteroids are used as an adjunct to the periocular injection, especially in severe or recalcitrant cases. We generally prefer using periocular corticosteroid injections to oral corticosteroids for several reasons. Periocular injection delivers a much higher concentration of corticosteroids to the eye than oral corticosteroids. There are fewer serious side effects with periocular corticosteroids. With oral corticosteroids, side effects include elevation of the serum glucose level, elevation of blood pressure, loss of bone density, weight gain, water retention, increased skin fragility, easy bruisability, and psychological changes. We generally do not recommend the prolonged use of oral corticosteroids in treating pars planitis.
Intravenous pulse corticosteroids are seldom used to treat pars planitis. Although intravenous pulse therapy with methylprednisolone has been used in the treatment of pars plantitis,66 we have never used intravenous corticosteroids as a first-line treatment of pars planitis, except in cases in which the patient also has MS and coincident optic neuritis.
Carbonic anhydrase inhibitors can be beneficial in the treatment of cystoid macular edema in pars planitis. Carbonic anhydrase inhibitors may be given orally or topically. Cox and coworkers67 have shown that oral acetazolamide and dichlorphen-amide are effective in treating cystoid macular edema in pars planitis. However, Whitcup and colleagues68 later reported no improvement in visual acuity in uveitis patients treated with acetazolamide for cystoid macular edema. Topical carbonic anhydrase inhibitors have not been tried in cystoid macular edema in pars planitis. One of the topical carbonic anhydrase inhibitors, dorzolamide, has been reported to be effective in improving the macular edema on the fluorescein angiogram in patients with retinitis pigmentosa.69 However, dorzolamide did not improve the visual acuity in these patients. The main disadvantage of carbonic anhydrase inhibitors is that macular edema frequently returns after cessation of the therapy. Carbonic anhydrase inhibitors may be useful in patients with very mild cystoid macular edema.
Cryotherapy of the inferior snowbank has been advocated.70–72 Some patients have entered remission and have had improved visual acuity after cryotherapy. However, cryotherapy usually is a temporizing measure, and the snowbanks usually return 3 to 6 months after treatment.70 Recently, Pulido and coworkers73 reported that peripheral retinal laser photocoagulation was effective in pars planitis in decreasing the use of corticosteroids, decreasing the vitreous inflammation, decreasing the neovascularization at the vitreous base, and improving cystoid macular edema. It seems reasonable to consider peripheral retinal laser photocoagulation in pars planitis patients who are not responding well to corticosteroids or who are not tolerating the side effects of corticosteroids.
Vitrectomy may be of benefit for cases of cystoid macular edema or persistent intraocular inflammation that fail to respond to corticosteroids.74,75 After a 3- to 4-month course of unsuccessful corticosteroid therapy, we might consider vitrectomy. From our experience, vitrectomy may be beneficial in treating recalcitrant vitreous inflammation and cystoid macular edema. However, the cystoid macular edema may take months to resolve after vitrectomy and often requires intensive corticosteroid therapy after surgery. Vitrectomy is also effective in removing persistent vitreous hemorrhage and vitreous debris. Epiretinal membrane peel can also be accomplished through vitrectomy.76 Although vitrectomy is useful, it has the potential complications of endophthalmitis, retinal tears, and retinal detachment, and often visually significant cataract develops after vitrectomy in patients with pars planitis.
In some patients with pars planitis, a favorable therapeutic response has been noted with the use of immunosuppressive agents77 such as chlorambucil,78,79 azathioprine,80,81 cyclophosphamide,82,83 and methotrexate.84,85 However, there is no clear evidence that traditional immunosuppressives hold any value when corticosteroids are ineffective. Steroid-sparing agents such as methotrexate and azathioprine are frequently substituted for corticosteroids in patients who require more than 3 to 6 months of high-dose (over 10 mg/day) prednisone. The long-term side effects of methotrexate and/or azathioprine may be less than those of corticosteroids. Immunosuppressive agents may be used cautiously in patients who have failed to respond to corticosteroid therapy and vitrectomy. Immunosuppressives in these patients, however, may need to be maintained indefinitely to keep the intraocular inflammation under control. The side effects of immunosuppressives are considerable, including bone marrow suppression, infertility, and organ toxicities. We recommend that if the ophthalmologist is unfamiliar with these drugs, an internist be consulted in monitoring the patient.
Cyclosporine has been reported to decrease the intraocular inflammation and improve macular edema in uveitis patients in whom corticosteroid therapy previously failed.86–89 However, in these patients, long-term use of cyclosporine is required. Nephrotoxicity occurs in about 50% to 75% of these patients, and the serum creatinine level must be monitored closely.89,90 Other side effects include gingival hyperplasia and hirsutism.89 We recommend the use of cyclosporine and other immunosuppressives only when the patient has failed to respond to corticosteroid therapy. Usually cyclosporine is used as a steroid-sparing agent to reduce the need for high-dose systemic corticosteroids.
Cataract surgery in pars planitis patients can be helpful. However, much attention must be paid to the amount of intraocular inflammation. We generally would not recommend cataract surgery unless the eye has been clear of active inflammation for at least 3 months. Exacerbation of intraocular inflammation, elevated intraocular pressure, and development of cystoid macular edema must be monitored closely after surgery. Fogla and coworkers91 reported a rate of 94% of visual improvement after cataract surgery in 52 eyes with intermediate uveitis. Michaelson and associates92 reported enveloping dense fibrous membranes (cocoons) in some patients with pars planitis and intraocular lenses. Some of these intraocular lenses had to be removed due to the density of the membranes and failure of the Yag laser to clear them. Low-grade postoperative inflammation can cause this problem, and patients must be monitored frequently for the first 3 to 6 months. Opacification of the posterior capsule tends to develop early and frequently in these patients, and laser capsulotomy may be required. We would proceed with laser capsulotomy only if the eye were uninflamed and cystoid macular edema were controlled.