While natural immunity is containing the antigen, acquired immunity (also known as specific immunity) is mounted to destroy the foreign substance. Acquired immunity is stimulated by exposure to foreign substances, is extremely specific for particular macromolecules, and increases in magnitude with each successive exposure.1 Extensive interaction occurs between natural and acquired immunity. Often, the type of acquired immunity developed by a host—critical in successful clearance of a pathogen—is directly influenced by signals from the innate immune system.
As our knowledge of the immune response increases, its complexity becomes more apparent. After generating an immune response to an antigen, the next important task is regulating that response. Although the immune response usually functions effectively, it occasionally goes awry and leads to autoimmunity.
GENERATION OF THE IMMUNE RESPONSE
A complex series of events is initiated when the immune system responds to an antigen. The first line of defense is natural immunity, which is present before exposure to antigen. Components of this branch of immunity include physical barriers, such as skin and mucosal membranes; cells, such as neutrophils, eosinophils, basophils, macrophages and natural killer (NK) cells; and soluble plasma proteins constituting the complement system.
Neutrophils, the major cell population in acute inflammation, and tissue macrophages respond rapidly to chemotactic stimuli and subsequently phagocytose and destroy foreign particles nonspecifically. Tissue mast cells and circulating basophils contain inflammation-inducing granules, the release of which is responsible for immediate hypersensitivity (allergic) reactions. Eosinophils, which also contain potent inflammatory granules, are known to function mainly in parasitic and helminthic infections. Additionally, the presence of eosinophils has been noted at sites of chronic allergy and inflammation. NK cells are large granular lymphocytes that kill target cells by osmotically lysing target cells, with exocytosed granules containing perforin and granzymes, and by inducing apoptosis (programmed cell death); this killing is not specific for any antigen and is categorized as part of natural immunity.1
While natural immunity initially “keeps the enemy at bay,” it directs the acquired immunity to deliver the definitive attack against the foreign invader. Furthermore, the acquired immune response focuses and directs natural immune mechanisms to eliminate the antigen. The generation of acquired immunity entails (1) the production and secretion of antibody (immunoglobulin) into the blood and other body fluids (i.e., humoral immunity), and (2) the generation of sensitized lymphocytes (i.e., cellular immunity).
Humoral immunity involves soluble and circulatory factors in the immune system, such as antibodies and complement proteins. Whereas antibodies are generally considered part of acquired immunity, the complement cascade participates in host defense by two different pathways. One pathway is activated by antigen-antibody complexes (classical) and the other is nonspecific (alternative), thus contributing to both the acquired and natural immune responses. Activation of either pathway leads to covalent binding of complement proteins to the microbial cell surfaces. Complement activation on the surface of a microbial cell promotes adherence of the microbe to a phagocytic cell that is competent at phagocytosing and killing the microbe. This process of “tagging” a cell with antibody or complement factors to facilitate phagocytosis is referred to as opsonization.
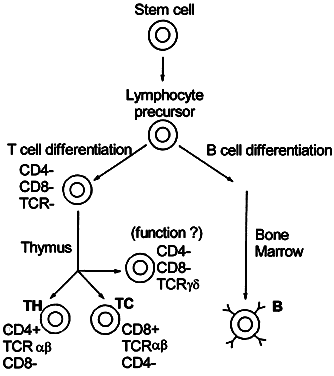
Cellular immunity is based on the function of the lymphocyte.2 Although lymphocytes are morphologically identical, they are functionally heterogeneous. They express an array of cell-surface molecules (markers), which define two major populations: T cells, B cells, and their subsets (Fig. 1).3
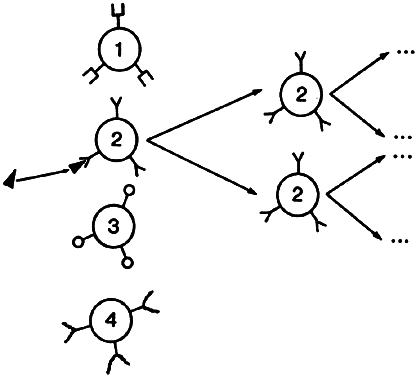
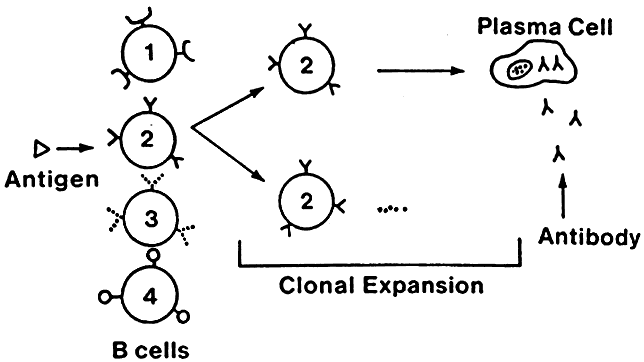
Lymphocytes interact with each other and with macrophages to allow selective expansion or suppression of specific populations (clones) of lymphocytes. Each lymphocyte clone has a unique receptor on its cell surface, which allows it to recognize and combine optimally with one antigen. Combination of this protein receptor with antigen results in the transmission of a signal across the cell membrane that activates the lymphocyte and results in selective proliferation and expansion of that lymphocyte clone (Fig. 2). Although numerous cellular interactions are involved in the generation of an immune response to antigen, our focus is on three distinct types: (1) antigen recognition by macrophages (phagocytic cells that play a key role in cellular immunity) and presentation to T cells (macrophage-T-cell interaction); (2) antibody production in response to antigen (T-cell-B-cell interaction); and (3) cytokine-mediated augmentation or inhibition of T-cell differentiation and proliferation (T-cell-T-cell interaction).
Macrophage-T-Cell Interaction
T-cell antigen recognition and activation depends on presentation of peptide antigen by specialized antigen-presenting cells (APCs). APCs include macrophages, B cells, and dendritic cells, which are accessory cells such as Langerhans cells in the skin or follicular dendritic cells in lymph nodes. All APCs digest proteins into peptide fragments and present these peptides within the clefts of special surface proteins coded by the major histocompatibility complex (MHC).
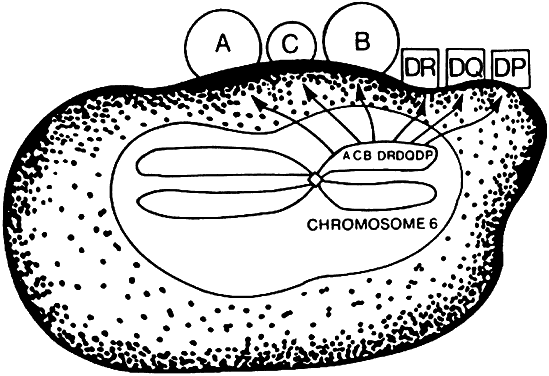
The MHC is a genetic segment on the short arm of chromosome 6 that codes for class I molecules (e.g., human leukocyte antigen (HLA)-A, HLA-B, and HLA-C) and class II molecules (HLA-DR, HLA-DP, HLA-DQ; Fig. 3) in addition to complement proteins and cytokines. Class I and II molecules were originally discovered as triggering T-cell responses leading to transplant rejection, hence the origin of the term histocompatibility.
The class II region, first identified in mice as the I region,4 codes for protein molecules expressed only on the cell membrane of macrophages, B cells, and dendritic cells. Class II-associated antigen presentation requires phagocytosis of extracellular antigens (e.g., bacterial proteins), followed by intracellular processing of the antigens. Activation of a major subset of T cells termed CD4+ helper T cells requires class II-associated antigen presentation; therefore, CD4+ T cells are called class II-restricted. (CD—cluster of differentiation—molecules are lymphocyte cell-surface markers; CD4+ designates helper T cells, and CD8+ identifies cytolytic T cells).
Class I molecules are expressed on almost all cells and are involved in presentation of endogenous antigens, such as tumor antigens and viral proteins, of which the latter are not phagocytosed but enter the cell by viral infection. CD8+ cytolytic T lymphocytes (CTL) are class I-restricted and on activation destroy the entire host cell harboring the foreign antigen.
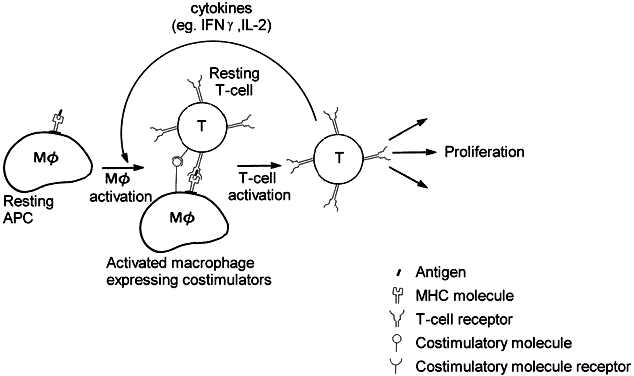
The manner in which MHC molecules interact with antigen or T cells has only been elucidated in the past decade. It has been shown that the MHC molecule binds antigen within its clefts and that the T-cell receptor (TCR) has regions that recognize the MHC complex and other regions that contact the foreign peptide (Fig. 4).5 The MHC-antigen complex must bind the antigen-specific TCR to achieve T-cell activation. Costimulatory molecules on APCs also bind with other T-cell membrane receptors during this APC-T-cell binding and may be responsible for the development of either activation or tolerance.1
Antigen recognition is followed by clonal proliferation and expansion of those T lymphocytes that are directed at the involved antigen. Several antigen-specific memory T cells are also generated during this process, enabling the host to mount a greater and faster response on future exposure to the same antigen. Upon activation, the T cell elaborates many soluble factors called cytokines, including interleukin-2 (IL-2). IL-2 is the major T-cell growth factor, acting both in an autocrine and in a paracrine fashion, stimulating growth of not only other CD4+ and CD8+ T cells but also B cells and NK cells. The potent immunosuppressant drug cyclosporin inhibits the production of IL-2 by activated T cells, thereby blocking proliferation and differentiation of immune cells. Cytokines are not unique to T cells but are secreted by many different types of cells, enabling the immune system to orchestrate inflammatory responses.
In particular, interferon-gamma (IFN-γ) and tumor necrosis factor (TNF) secreted by T cells activate macrophages by setting off a cascade of upregulatory events, rendering the macrophage a more powerful and efficient killer of intracellular organisms. For example, on activation, the macrophage is induced to produce large amounts of nitric oxide, which is thought to inhibit bacterial and viral replication. While the proliferation of microorganisms is inhibited, potent proteases are released within the phagolysosomes, thereby destroying the pathogens more effectively. Also, angiogenic factors are released, such as fibroblast growth factor, which initiates endothelial cell migration and proliferation and thus tissue repair.
T-Cell-B-Cell Interaction
B-cell and helper T-cell cooperation entails activation and proliferation of both subsets of lymphocytes. As described above, T-cell activation requires antigen presentation, and B cells can serve well as APCs. Once antigen is recognized by a surface immunoglobulin on a B cell, the B cell is activated. The antigen is subsequently internalized, processed, and presented by the B cell in the context of a class II MHC molecule. The advantage of B cells over other APCs is that they are able to present antigen at a 104- to 106-fold lower concentration.1
Binding of a T cell to an antigen-presenting B cell leads to T-cell activation and release of IL-2, among other cytokines, promoting antigen-specific B-cell and T-cell proliferation. Binding of CD40, a B-cell surface receptor, to its complementary CD40 ligand on the activated T cell is also necessary for B-cell activation, differentiation, and survival. Other cytokines elaborated as a result of T-cell activation (e.g., IL-4, IL-5, transforming growth factor beta [TGF-β], and IFN-γ) serve to enhance antibody secretion and isotype switching.1
The production of antibody against most antigens requires this synergistic cooperation of helper T cells and B cells. The omission of either population results in limited or no antibody production. This is true in both the primary (i.e., on initial antigen exposure) and secondary (i.e., on subsequent antigen exposure) antibody responses.
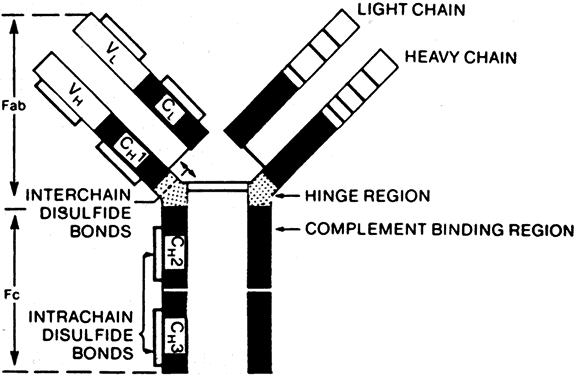
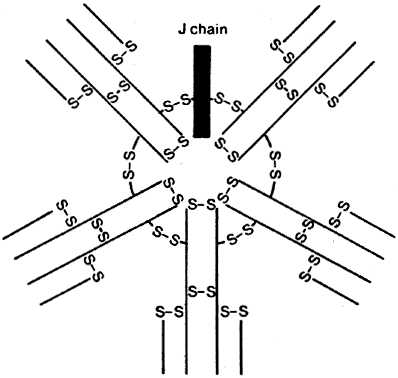
B cells respond to a wider variety of antigens than T cells, including polysaccharides, lipids, and proteins. The selective activation of B cells is followed by differentiation into immunoglobulinsecreting cells (i.e., plasma cells; Fig. 5). Memory B cells are also produced in this process of differentiation for a response of greater magnitude and speed on future encounter with the same antigen. Immunoglobulins are an important component of the response of the immune system to invasive pathogens. The basic structure of an immunoglobulin molecule consists of two heavy and two light chains, each composed of specific amino acid sequences (Fig. 6). There are five different classes (or isotypes) of immunoglobulin molecules based on the amino acid sequence in the constant region of their heavy chain (IgG, IgM, IgA, IgE, and IgD). The IgG molecule has the structure depicted in Fig. 6. In contrast, the IgM molecule is a pentamer, consisting of five such units linked by a joining (J) chain (Fig. 7). The IgA molecule is most frequently a dimer, also joined by the J chain.
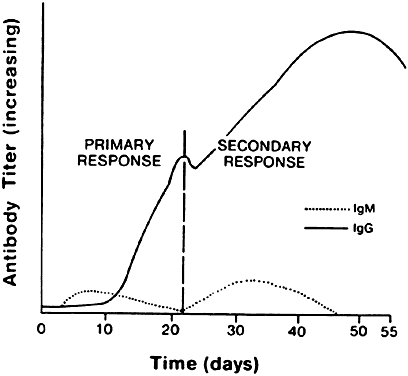
The isotype of antibody that is produced can be important diagnostically. After initial contact with antigen, the immune response produces antibody in what is called the primary response (Fig. 8). The first class of antibody produced is IgM. Only a low titer of IgM antibody is produced, however, and it rapidly declines to undetectable serum levels. During the second week of the immune response, an IgM to an IgA, IgE, or IgG isotype switch occurs, depending on the cytokine produced by the helper T cell, and high titers of the new isotype antibody are produced in the plasma. These titers persist long after the onset of the immune response. When the immune response again encounters the same antigen, a secondary antibody response occurs. The main antibody produced by the memory B cells is either IgA, IgE, or IgG, which reaches even higher serum levels and persists even longer. In contrast, IgM antibody titers appear transiently, remain much lower, and rapidly return to undetectable levels.
T-Cell-T-Cell Interaction
The interplay between subsets of T cells is achieved through secretion of cytokines, which modulate the immune response. For example, the immune response to antigen can result in the generation of CD8+ CTLs with the ability to kill virus-infected host cells or allogeneic transplanted cells (i.e., transplanted cells from genetically different individuals of the same species). Optimal generation of CTLs from their precursors (pre-CTLs) requires cytokine release from helper T cells.
Initially, pre-CTLs bind peptide antigen expressed in the context of class I MHC molecules on the surface of target cells and are thus activated. Additional ligand-receptor interactions between pre-CTLs and target cells, in the presence of the cytokines IL-2 and IFN-γ, subsequently lead to full activation and differentiation of pre-CTLs to CTLs capable of killing target cells. IL-2 and IFN-γ, both of which are necessary for the full activation and differentiation of CTLs, are produced mainly by neighboring helper T cells, which are concurrently activated in response to the viral infection or allogeneic graft. Helper T cells, however, are activated by a different peptide sequence that binds to class II MHC molecules on APCs. Dendritic cells especially have been implicated in presenting foreign proteins expressed by viruses or allogeneic grafts by endocytosing fragments of apoptotic cells that express these proteins. Therefore, an intimate cooperation between class I-restricted CD8+ T cells (i.e., pre-CTLs) and class II-restricted CD4+ helper T cells leads to the development of CTLs capable of recognizing and killing target cells that express foreign proteins.
This accessory ligand-receptor conjugation between the target cell and the antigen-specific CTL is required for efficient killing. Once this conjugate is formed, CTLs deliver their lethal hit mainly by granule exocytosis (e.g., perforin, a membrane pore-forming protein; and granzymes, enzymes containing reactive serines at their active site), leading to apoptosis and osmotic lysis.
Many apoptotic mechanisms in the immune system have been linked to the interaction of Fas ligand, a cell-surface protein on the killer cell, with its receptor Fas (also known as CD95) on the target cell. The binding of this receptor to its ligand triggers a cascade of intracellular protein-protein interactions and proteolytic activities, culminating in apoptosis of the target cell. This pathway is thought to be more important in immune regulation—that is, for controlling excessive lymphocyte activation especially against self-antigens—than for CTL functions.1,6
REGULATION OF THE IMMUNE RESPONSE
Because multiple cell-cell interactions occur during the generation of an immune response, it is necessary to regulate these interactions so that the net effect is appropriate, minimizing the inadvertent destruction of innocent host bystander cells.
Multiple homeostatic controls exist to achieve immunoregulation, of which we briefly describe the principal mechanisms. So far, clonal expansion has been considered in the context of selection by antigen. As such, the regulation of clonal expansion can be achieved by antigen removal by secreted antibody or T cells. The depletion of antigen for lymphocyte receptors serves as negative feedback for antigen-driven clonal expansion. Thus, only memory cells remain, which require subsequent activation by antigen for regeneration of a full response.
Immunologic tolerance is an important mechanism for immune regulation, applicable to both B and T lymphocytes. Studies have shown that tolerance is antigen-specific and thus likely achieved by either deletion or inactivation of specific B- or T-cell clones (i.e., clonal deletion or clonal anergy). Immature lymphocytes are more susceptible to induction of tolerance than mature, functional cells. In the thymus, immature T cells are thought to be exposed to self-antigens; if a T cell recognizes any self-antigen, it is eliminated by apoptosis. This mechanism of tolerance is referred to as clonal deletion because it involves cell death. The purpose of this central tolerance (central because it occurs in the thymus) is the prevention of autoimmunity.1
Under special conditions, tolerance may be induced in mature lymphocytes, away from the central lymphoid tissues (peripheral tolerance). For example, a large concentration of foreign polysaccharides injected intravenously does not stimulate a B-cell response; even subsequent exposure to immunogenic doses of the antigen fails to activate B cells. Peripheral CD4+ T-cell tolerance has also been achieved in various ways, including high-dose intravenous administration of proteins without adjuvant (high-dose tolerance), repeated low-dose exposure to proteins without adjuvant (low-dose tolerance), and oral administration of proteins (oral tolerance). T-cell tolerance is generally induced at lower concentrations of protein than is B-cell tolerance and lasts longer. Moreover, without CD4+ T-cell help, the B-cell response to low levels of protein antigen is less. The mechanisms involved in peripheral tolerance are clonal anergy as well as clonal deletion—the former referring to functional inactivation without cell death.1
Because the antigen receptor on lymphocyte cell membranes is the only clonally distributed structure, it is likely involved in cell regulation. This can occur through either cell-receptor interaction with antigen or with factors directed at the antigen receptor itself (i.e., idiotype-anti-idiotype recognition). The precise details of the idiotypic-anti-idiotypic networks of immunoregulation are not discussed here because it has a more theoretic basis with insufficient experimental evidence.
Some evidence has been provided for immune regulation mediated by suppressor T cells. Despite repeated studies and attempts to purify these cells, however, not much advancement has been made. Therefore, one thought is that perhaps a series of suppressor mechanisms exist instead of a discrete class of suppressor T cells.1 For example, transforming growth factor-beta (TGF-β) effectively inhibits T-cell and B-cell proliferation; therefore, cells that secrete significant amounts of this cytokine may function as suppressor cells.
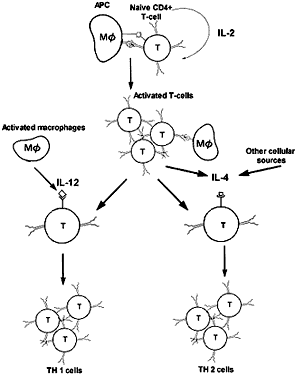
Similarly, different types of immune responses may be suppressed. CD4+ T cells have been further subclassified as T helper 1 (TH1) and T helper 2 (TH2) cells. TH1 cells direct the immune reaction toward a cell-mediated response through expression of IFN-γ, IL-2, and TNF-α; TH2 cells elaborate IL-4, IL-5, and IL-10, leading to IgE-mediated humoral immunity. Interestingly, IFN-γ inhibits the IL-4-mediated B-cell isotype switch to IgE; conversely, IL-4 inhibits IFN-γ-mediated macrophage activation. The trigger for the TH1 response has been shown to be IL-12 secreted by the activated macrophage (Fig. 9).1
In certain conditions, however, the desirable immune response becomes suppressed, leading to a detrimental outcome. For example, a TH2 response in leprosy is associated with chronicity and greater host-tissue destruction, whereas a TH1 response theoretically would be more effective. One explanation for the tendency toward a TH2 response may be as follows. IL-4 is initially produced in small amounts by the T cell at the time of its initial activation, and its concentration increases when high concentrations of antigen persist. If this is combined with the absence of IL-12 secretion, a TH2 response will predominate. Genetic factors have also been implicated in the predominance of one type of response over the other.1