SKIN TESTING FOR DELAYED HYPERSENSITIVITY
Intradermal injection of 0.1 ml of soluble antigens prepared from a number of infectious agents can be performed in the office during the initial visit. The most useful test antigen is the tuberculin purified protein derivative (PPD). The standard dose is 5 TU (US tuberculin units) or intermediate strength, equivalent to 0.0001 mg (Aplisol, Parke-Davis, Morris Plains, NJ). Current lots of PPD are standardized for biologic activity against PPD-Seibert (PPD-S), a reference bulk lot prepared in 1940. Second strength (250 TU) can be given to patients who do not react to an initial 5-TU injection, whereas first strength (1 TU) should be given to patients who the clinician suspects may have a strong reaction and are therefore at risk for sloughing and ulceration at the injection site (Tubersol, in first and second strength, available through Squibb/Connaught, Princeton, N J). There is no evidence of booster effects or repeated skin testing leading to conversion from negative to positive.4 Intracutaneous injection, the Mantoux test, is the most reliable test available. Injection of 0.1 ml of PPD antigen into the volar or dorsal surface of the forearm is made just below the skin surface with a short (1/2-inch) No. 27 gauge needle, bevel upward, to produce a discrete 6- to 10-mm wheal or bubble. The use of insulin syringes with intrinsic needles will avoid repeated waste of antigen extract in the needle hub of standard tuberculin syringes. If no bubble appears due to a deep injection, the test should be immediately reapplied at a site at least 5 cm away. Skin tests are read 48 to 72 hours following injection. Induration measuring 10 mm or more is considered positive: this indicates a previous infection with Mycobacterium tuberculosis, although previous infection with M. bovis or photochromogenic mycobacteria may also produce a positive result. Patients may also develop coexistent erythema, a wheal and flare that usually fades after 12 to 18 hours, which is evidence of an immediate hypersensitivity to the same test antigen. Uveitis patients with induration less than 10 mm, or even erythema alone, have had favorable clinical responses to oral antituberculous therapy.5 On the other hand, patients with proven ocular tuberculous infection have had insignificant6 or negative7 PPD skin tests.
Other commercially available skin test antigens include histoplasmin (Parke-Davis, Morris Plains, N J) and coccidioidin (Spherulin 1:100, Berkeley Biologicals, Berkeley, CA). Although the use of histoplasmin skin testing in uveitis is no longer universally recommended, greater than 80% of patients with presumed ocular histoplasmosis syndrome have positive skin tests.8 Some authors believe that histoplasmin skin testing may increase the danger of activating a macular lesion in susceptible patients.
Skin test reagents for other infectious diseases are not commercially available and must be obtained through individual laboratories actively engaged in production and purification of the specific antigen. These include brucellosis, tularemia, blastomycosis, toxoplasmosis, nontuberculous mycobacterial disease, and cryptococcosis antigens, all of which are subject to strict safety controls limiting their use.9 Control antigens to which normal, presumably exposed, adult patients should react, include Candida extract (Dermatophyton O, 1: 100, Hollister-Stier, Spokane, WA), Trichophyton, mumps, and streptokinase-streptodornase (Lederle Diagnostics, Pearl River, NY).
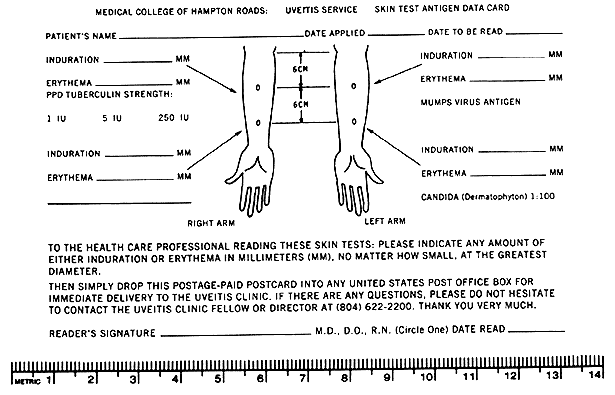
Patients with sarcoidosis and a number of other conditions (Table 3) are often anergic to all skin test materials.10 In patients taking corticosteroids, a negative test should be repeated or delayed until the medication is discontinued because systemic corticosteroids may reduce or eliminate all skin test reactions. A positive test, however attenuated, is still significant in patients on oral corticosteroid therapy. A self-addressed postcard clearly diagramming the position and dosage of all skin tests applied can be expeditiously returned after 48 hours by patients who do not have convenient access to the uveitis clinic (Fig. 1). Skin tests should be read only by health care personnel who understand diameter measurements of erythema and induration.
TABLE 3. Clinical Conditions Associated With Anergy
Technical Errors in Skin Testing
Improper dilutions of test antigens
Bacterial contamination
Exposure to heat or light
Adsorption of antigen to container walls
Faulty injection: too deep or leaking
Improper reading of reaction
Immunologic Deficiency
Congenital cellular immunodeficiency:
Thymic/parathyroid aplasia (DiGeorge's syndrome)
Mucocutaneous candidiasis
Congenital combined humoral and cellular deficiencies:
Ataxia-telangiectasia
Nezelof's syndrome
Severe combined immunodeficiency
Wiskott-Aldrich syndrome
Acquired:
Acquired immunodeficiency syndrome
Sarcoidosis
Chronic lymphocytic leukemia
Hodgkin's disease and lymphomas
Carcinoma
Immunosuppressive medications
Rheumatoid diseases
Uremia
Alcoholic and biliary cirrhosis
Surgery
Infections
Influenza, mumps, measles and viral infections
Viral vaccinations
Typhus
Miliary and active tuberculosis
Disseminated mycotic infection
Lepromatous leprosy
Scarlet fever
(Adapted from Heiss LI, Palmer DL: Anergy in patients with leukocytosis. Am J Med 56:323, 1974.)
Since a positive reaction to skin tests requires a succession of immunologic processes, a positive control skin test rules out a major defect in cell-mediated immunity. Bacille Calmette-Guérin (BCG) has been used in the past to test a patient's ability to react to a new antigen, but it should not be used in immunocompromised patients. Although BCG vaccination is routinely recommended in some European and Third World countries, it is given in the United States only to PPD-negative contacts of ineffectively treated or persistently untreated cases and other unusually high-risk groups. Another approach to rule out anergy is skin sensitization to dinitrochlorobenzene (DNCB) or dinitrofluorobenzene (DNFB) by one cutaneous application of a high concentration of the substance, usually on the volar aspect of the forearm. Three weeks thereafter a much smaller dose can be used to confirm sensitization.11 Virtually all normals can be sensitized in this manner. More advanced techniques to assess lymphocyte function include in vitro lymphocyte stimulation testing utilizing various antigens compared with known mitogen controls,12 assays for interleukins and other cytokines, as well as flow cytometry and cell sorting with fluorochrome conjugated antibody labels for a variety of leukocyte subset surface markers.13
Approximately 80% of patients with sarcoidosis react positively to skin testing with the Kveim antigen. This antigen is prepared from human sarcoid granulomas, is difficult to obtain, requires several months of incubation followed by a biopsy at the injection site for histologic verification, does not have absolute specificity, and entails a risk of hepatitis or retrovirus transmission. False-positive and false-negative results may occur, limiting its usefulness.
BEHÇET'S SKIN PUNCTURE TEST
Patients with active systemic Behçet's disease show increased dermal sensitivity to needle trauma, owing to their unusual leukotactic tendency. The appearance of a pustule 24 to 36 hours after an intradermal needle puncture, with or without the injection of 0.1 ml of normal saline, is almost diagnostic for Behçet's disease.14 Erythema and infiltration may appear within a few hours, and occasionally a microabscess can be produced. This test may not be positive in patients with ocular manifestations when the systemic disease is in remission. No universal agreement on the usefulness of this test exists because only 10% of Behçet's patients demonstrate a positive response.15
MACULAR FUNCTION AND THE PHOTOSTRESS TEST
Macular edema is a common complication of intraocular inflammation and can lead to the formation of irreversible cysts and macular holes. Early macular disease can be detected by progressive loss of visual acuity, Amsler grid testing, tangent screen examination, careful contact lens or 90-diopter lens biomicroscopy, and fluorescein angiography. Maximal visual acuity after shining a bright light into the eye of a normal person recovers in less than 180 seconds. The photostress test is considered abnormal when there is marked asymmetry, or if the recovery time is greater than 180 seconds in either eye. The photostress test is extremely sensitive and may detect a maculopathy not demonstrable by any other measure. The recovery time is not prolonged in patients with optic nerve disease.16
TEAR FUNCTION TESTING
Aqueous tear deficiency states associated with uveitis include sarcoidosis, Sjögren's syndrome, and other systemic connective tissue diseases. Schirmer's tear strip testing or tear lysozyme levels can be used to quantify aqueous tear production. Tear lysozyme concentration can be measured with Micrococcus lysodeikticus bioassay plates, spectrophotometric, viscometric, turbidimetric, and immunologic methods. In contrast to classic keratitis sicca, patients with sarcoidosis and aqueous tear deficiency may have normal or elevated tear lysozyme levels.17
Other tear enzymes, such as lactoferrin, can be used to estimate tear production (Lactoplate, Eagle Vision, Memphis, TN). A tear-soaked filter paper disc is used in office-based tests in order to measure a standardized tear volume.18 Patients with active ocular sarcoidosis, even in the absence of systemic disease, are likely to have elevated tear angiotensin-converting enzyme (ACE) activity.19 Tear ACE levels provide a noninvasive test for diagnosing and monitoring this disease. Cytomegalovirus has been recovered on occasion from the tears of patients with active systemic infections.
CONJUNCTIVAL BIOPSY
A conjunctival biopsy containing discrete, noncaseating, subepithelial granulomas can be diagnostic for sarcoidosis. Disagreement exists as to whether there is value in obtaining a random, or “blind,” conjunctival biopsy to rule out sarcoidosis. Some reports demonstrated that 28% of random specimens were positive in selected patients who were suspicious for sarcoidosis without obvious conjunctival lesions.20,21 Conjunctival biopsy is more likely to be diagnostic when granulomas are present in the inferior fornix, a large volume of tissue is removed, and serial sections are carefully examined. Random conjunctival biopsies yielded noncaseating granulomas in 55% of patients who already had or were later documented to have systemic sarcoidosis by positive biopsy from another site.22 In the usual clinical setting, however, it is superfluous to obtain a conjunctival biopsy from a patient who is already known to have sarcoidosis.
Conjunctival biopsy is much safer than bronchoscopy or even lacrimal gland biopsy, and it is reasonably specific when interpreted along with other clinical and laboratory data.23 Biopsy of cutaneous lesions or minor salivary glands24 is also safe and may be useful in suspected sarcoidosis.25
AQUEOUS PARACENTESIS
Occasionally, anterior chamber fluid can provide information essential in establishing the etiology of a patient's uveitis. Paracentesis may prove useful if specific antibodies to infectious agents, such as Herpes simplex, Toxoplasma, Toxocara, Leptospira, Treponema pallidum, or Mycobacterium tuberculosis are found in greater concentration in the aqueous humor than in the serum. Angiotensin-converting enzyme levels in the aqueous may be elevated in sarcoidosis.26 The value of aqueous ACE determinations may be limited by poor specificity due to the presence of high intrinsic ACE levels in normal ocular tissues, particularly retinal vessels.27 Aqueous eosinophilia is seen in ocular toxocariasis28 or in cysticercosis29 associated with hypopyon. In retinoblastoma, the aqueous to plasma lactate dehydrogenase (LDH) ratio is often elevated to 50:1 or more, whereas the ratio is less than 1:1 in pseudoretinoblastoma patients.30 Aqueous cytology may also be diagnostic for retinoblastoma, particularly when associated with pseudohypopyon, but the danger of extraocular seeding must always be considered when tapping eyes with suspected tumors.
In phacoanaphylactic endophthalmitis, anterior chamber exudates consist of polymorphonuclear granulocytes and monocytes.31 This exudate can provoke secondary open-angle glaucoma.32 Phacoanaphylactic endophthalmitis may occur after accidental trauma, surgical lens discission, extracapsular cataract extraction, or spontaneous lens rupture in hypermature cataract. Phacolytic open-angle glaucoma occurs when macrophages and amorphous lens material obstruct the trabecular meshwork.33 Phacolytic glaucoma may follow traumatic or inapparent lens injury. A milder form of lens-induced uveitis probably occurs in all cases of lens trauma and may be granulomatous or nongranulomatous. This macrophage response, also called phacotoxic or phacogenic uveitis, corresponds to a foreign body reaction. Aqueous aspirates may contain macrophages and epithelioid cells laden with cytoplasmic periodic acid-Schiff (PAS) positive granules, as well as plasma cells and lymphocytes. The spectrum of aqueous cytology may assist the ophthalmic pathologist in providing a diagnosis of suspected lens-induced uveitis.
Cytologic specimens taken from body fluids are best fixed by rapid air drying, or with absolute methanol or 80% ethyl alcohol. Staining with Wright's, Giemsa (Eastman Kodak, Rochester, NY), or Diff-Quik (American Scientific Products, McGaw Park, IL) will allow maximal resolution of cellular characteristics. In viral infections and chronic hypersensitivity reactions, mononuclear cells predominate. In acute reactions, such as HLA-B27 related and Behçet's syndrome uveitis, polymorphonuclear leukocytes abound. A Gram stain should be performed when infection is suspected. A Papanicolaou stain may best identify herpetic intranuclear and intracytoplasmic inclusions.34 When a paracentesis is performed to rule out intraocular spread of a known neoplasm Such as lymphoma or leukemia, the specimen should be compared with a diagnostic bone marrow or lymph node biopsy. This allows more certain matching of the neoplastic cell types.
In Fuchs' heterochromic iridocyclitis, progressive iris atrophy is often accompanied by abnormal vessels in the angle. An anterior chamber tap may therefore produce a filiform hemorrhage, known as Amsler's sign. This test is not clinically productive and is not recommended, especially since the diagnosis is usually readily apparent from the characteristic constellation of physical findings. In addition, simple gonioscopy will adequately demonstrate these fine chamber angle vessels.
Aqueous paracentesis is a safe office procedure when Close attention is paid to patient control and education, meticulous aseptic technique, and respect for the iris and lens structures. Topical 4% lidocaine (Xylocaine), adequate assistance, a wire speculum, povidone-iodine (Betadine) irrigation, ocular control with a fine-toothed forceps at the temporal limbus, and a No. 30 gauge needle fitted to a 1-ml plastic tuberculin syringe will minimize potential hazards. The tap can be done at the slit lamp Or with surgical loupes with the patient in the supine position. Afterward, the eye is patched for several hours, and topical antibiotics prescribed four times a day over the following 4 days. Patients are always rechecked 24 to 48 hours postoperatively.
Vitreous aspiration in the outpatient setting can also be safely performed in selected circumstances. Aspiration through the pars plana with a No. 18 gauge blunt-tipped needle following retrobulbar anesthesia can be done with the patient in a semireclining position or at the slit lamp. When therapeutic antibiotic or corticosteroid injections are anticipated following the vitreous tap, a three-way Luer-Lok stopcock avoids the problem of needle replacement. Care should be taken to constantly monitor the depth of needle penetration in order to avoid retinal or optic nerve injury. Indications are the same as for diagnostic surgical vitrectomy.
FLUORESCEIN ANGIOGRAPHY
Angiography is useful in delineating macular edema, vasculitis, disc edema, inflammatory foci, or old scars found in uveitis. One must differentiate leaking fluorescence, indicative of inflammatory activity, from that which radiates through thinned pigment epithelium (transmitted fluorescence), which fades fairly rapidly over 10 to 30 minutes and does not indicate inflammatory activity. Transmitted fluorescence may be seen at the earliest stages of uveitis35 or represent old, inactive, destruction lesions. Fluorescence that persists may arise from stained sclera or from a pool of fluorescein in the choroid or under the sensory retina.36 Areas of chorioretinitis due to toxoplasmosis and toxocariasis are often severe and demonstrate prominent fluorescence, which may increase with time (staining). In the early stages, however, the fluorescence from the choroid may be obstructed by the opaqueness of the retinitis (blocked fluorescence). The nonfenestrated retinal vessels resemble blood vessels in the central nervous system in that their vascular endothelium is impermeable to fluorescein dye.37 In contrast, the choroidal vessels, like blood vessels outside the central nervous system, are permeable to the dye.
Visualization of the ciliary processes is possible with a special three-mirror contact lens with a ridge to depress the ciliary body region. Following intravenous injection, fluorescein can be seen to enter the posterior chamber through the prebasal cortex of the vitreous body. Thus, inflammation of the cornea ciliaris is relatively easily recognized.38 Even without this lens, leakage from the corona ciliaris can be detected if the anterior part of the vitreous becomes greenish after 10 to 60 minutes. Direct observation obviates the problem of differentiating true from pseudofluorescence on black-and-white photographic film.39
Intravenous fluorescein may also aid in anterior segment examination. In iritis, the anterior chamber becomes dark green. In cyclitis, the retrolental space may fluoresce. The fundus camera may also demonstrate accentuated fluorescence of iris vessels, especially in blue irides40 during inflammatory episodes. Iris angiography may also detect early vascular damage in Fuch's heterochromic iridocyclitis,41 recent maturity-onset diabetes, or hyperlipoproteinemia.42 Fluorescein injections may also be used to delineate foci of episcleritis and scleritis and to define active areas of clinically suspicious necrotizing scleritis.43
The standard intravenous dose is 5 ml of 10% fluorescein. Within 3 to 5 minutes, the fluorescein is equally distributed throughout the blood. It is eliminated, largely by way of the kidney, in about I hour. Toxicity is rare, but nausea, the most common side-effect, occurs within 15 to 30 seconds after injection and subsides within 1 minute. Nausea and vomiting can usually be prevented by an intramuscular injection of 25 mg promethazine hydrochloride (Phenergan). Pyrexia has been observed after administration of at least one batch of fluorescein as a result of the incorporation of pyrogens. The estimated incidence of reactions to fluorescein is 0.6%, of which most are allergic, and only one fourth are severe. Reactors may develop itching and hives, wheezing with laryngeal edema, or anaphylactic shock. One reported patient died of a myocardial infarction several hours following a severe reaction.44 Oxygen and an emergency cart should always be available whenever fluorescein is administered.45 Patients who are known to develop urticaria following fluorescein can be pretreated with antihistamines if angiography is judged essential and anesthesia personnel are readily available. If allergic hypersensitivity is suspected, an intradermal skin test with 0.1 ml of fluorescein is suggested. Extravasated fluorescein causes immediate severe pain that lasts only a few minutes and is somewhat relieved by application of an ice pack. Rarely, skin sloughing46 or eczematous dermatitis47 may result.
VISUAL FIELDS
Visual fields are especially useful in differentiating heredodegenerative disease from uveitis. For example, primary retinitis pigmentosa has a different field than the bone spicule fundus pigmentation seen in luetic uveitis. Because many uveitic syndromes are associated with neurologic disease, a visual field may also be helpful in distinguishing neurologic papillitis from juxtapapillary retinitis. Since many uveitic entities are associated with secondary glaucoma, attention should be given to the possibility of progressive visual field loss.
ULTRASONOGRAPHY
Ultrasonography is noninvasive and most useful when the media are opaque. This test can characterize inflammatory foci, such as choroidal thickening, uveal tumors, orbital masses or infiltrates, intumescent or dislocated lenses, vitreous opacities, cyclitic membranes, choroidal or retinal detachments, some foreign bodies, and posterior scleritis.
Sound waves with frequencies greater than 18 KHz produce echoes as they strike interfaces between acoustically different structures. Reflected sound waves are converted into an electric potential by a piezoelectric crystal and displayed on a cathode ray tube. The A-scan mode provides a one-dimensional image of acoustic density and is useful as an aid to interpretation of the two-dimensional B-scan. The immersion method of ultrasonography allows greater resolution of anterior segment structures, more flexibility in the selection of transducer frequencies, and consecutive serial sections of the globe. Contact ultrasonography is more convenient and portable, requires less expensive equipment, and allows the examination of infants without anesthesia or direct compression of the globe.
ELECTRORETINOGRAPHY AND ELECTRO-OCULOGRAPHY
Electroretinography (ERG) measures the action potential evoked by light stimulation of the retina. Because this is a mass response the reading may be normal when retinal lesions are focal. The test is useful in detecting disease with extensive retinal pathology, such as central retinal artery or vein occlusion, retinal detachment, hypovitaminosis A, siderosis, diabetic retinopathy, anemia, chloroquine toxicity, hyperthyroidism, retinitis pigmentosa, acute diffuse chorioretinitis, and retinal destruction seen with Behçet's syndrome, cytomegalovirus retinitis, or acute retinal necrosis. Electroretinography is especially useful with opaque media and may signal retinal involvement before the clinician can make a diagnosis.48 Most studies indicate that there is no specific electroretinographic depression of diagnostic value in uveitis, although there is evidence that electroretinographic changes do correlate well with clinical and morphologic alterations. Corticosteroid administration may actually enhance electroretinographic responses.49
Electro-oculography (EOG) measures the standing potential between the positive cornea and the negative retina. A decrease in the ratio between the response in the light- and dark-adapted retina indicates a disorder of the retinal pigment epithelium. These electrical changes are seen in chloroquine retinopathy, diabetic retinopathy, siderosis retinae, flecked retina, vitiliginous maculopathy, and shallow retinal pigment epithelial detachments.