RADIAL KERATOTOMY
In 1938, Kokott described a circular ligament of the cornea2 that when cut via radial corneal incisions would cause bulging of the peripheral cornea. This peripheral bulge would induce a central flattening of the cornea, reducing its dioptric power and eliminating myopia. The principle of radial keratotomy (RK) was based on these findings. A Dutch ophthalmologist, Lendert J. Lans, is credited with developing the idea of partial-thickness incisional keratotomy.3 He constructed detailed drawings of the various forces acting on the cornea after incisional keratotomy. His work resulted in the understanding that the depth of a corneal incision was important in determining the extent of corneal curvature change. Despite Lans's significant advances, practical use of incisional keratotomy was not developed until the late 1930s.
Tutomo Sato experimented with posterior and anterior corneal incisions to induce corneal alterations in keratoconus patients. In his report of 281 eyes, Sato and colleagues showed a 3.00-diopter (D) reduction of corneal power using incisions created in the posterior corneal surface.4 It was not until 20 years later, with the discovery of the importance of the corneal endothelium, that the complications associated with his posterior incisions were discovered. A follow-up study of Dr Sato's patients found that 75% had developed significant corneal edema by 20 years after surgery.5
Because of the Sato experience, many ophthalmologists were skeptical of the popular incisional keratotomy procedures first developed by Svyatoslov Fyodorov in Moscow. Fyodorov reported excellent results but produced few published reports.6 When Leo Bores performed the first RK procedure in the United States in 1978, a strong public desire for the procedure ensued. Despite the lack of adequate peer-reviewed literature, RK became the first popular refractive surgery procedure. However, US physicians remained skeptical, demanding to see peer-reviewed data before accepting the newest cure for myopia.
Subsequently, the National Eye Institute sponsored the Prospective Evaluation of Radial Keratotomy (PERK) Study beginning in 1980 to evaluate the effectiveness and safety of RK. The PERK study was a multicenter, controlled study of patients who underwent RK. In the 19 years since its inception, the PERK study has served as the authority on the long-term results of RK and serves as a model for future prospective evaluations of refractive surgery. As the follow-up of the PERK study patients continued, the principal investigators identified several complications. We have divided these complications associated with radial keratotomy into intraoperative, postoperative, refractive, and anatomic.
INTRAOPERATIVE COMPLICATIONS
The initial work of Sato, Kotoff, and Fyodorov revealed that the incisions created for incisional keratotomy must be deep enough to induce maximum curvature change. Without the aid of accurate determination of the thickness of the corneal stroma and the depth of each radial incision, surgeons would inadvertently enter the anterior chamber. Two classes of perforations were then identified.7 Microperforations were classified as those that did not compromise the anterior chamber. Macroperforations, in contrast, led to a flat anterior chamber and required urgent repair. Early studies reported perforation rates of nearly 40%, but all were microperforations.8 Theoretically, microperforations increase the risk of endophthalmitis and endothelial cell damage.
To decrease the risk of both micro- and macroperforations, surgeons began to use diamond blades with adjustable blade lengths. The depth of the blade can be accurately set using a microscope mounted on a gauge block. In addition, ultrasound pachymetry led to more accurate measurements of corneal thickness. Surgeons could measure the corneal thickness, set the appropriate depth for the incisions, and be confident that the blade would not penetrate the anterior chamber. In the PERK study, four measurements of central corneal thickness were obtained. The diamond blades were set to 100% of the thinnest measurement, and the incisions were not redeepened with a second pass of the blade.
Perforations were also more prevalent with certain incision techniques. The original technique (Russian) described by Fyodorov used a centripetal (limbus to clear zone) pass of the blade. This technique would create its deepest point near the corneal apex, the thinnest portion of the cornea. In the PERK study, the American technique was used, creating radial incisions with a centrifugal (optical zone to limbus) stroke. The American-style incision is believed to have less chance of creating a perforation, because the central portion of the incision was the shallowest. Because of these refinements, the rate of microperforation in the PERK study was 2.27%,9 and there were no perforations that required surgical repair.
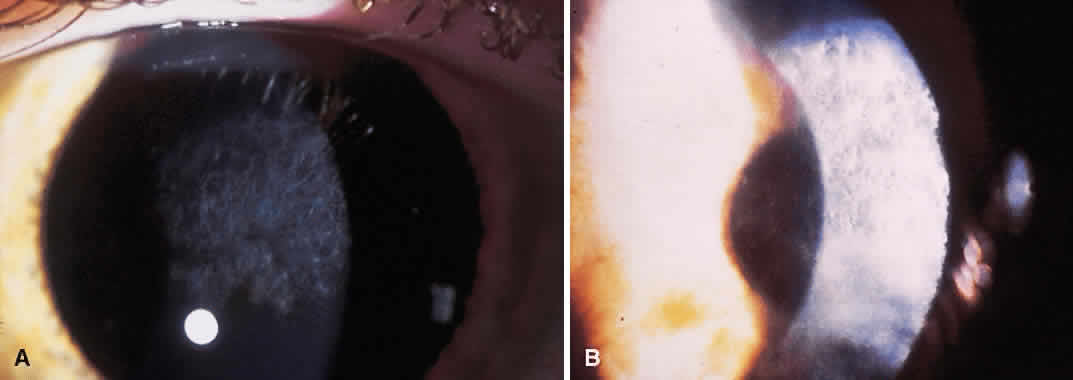
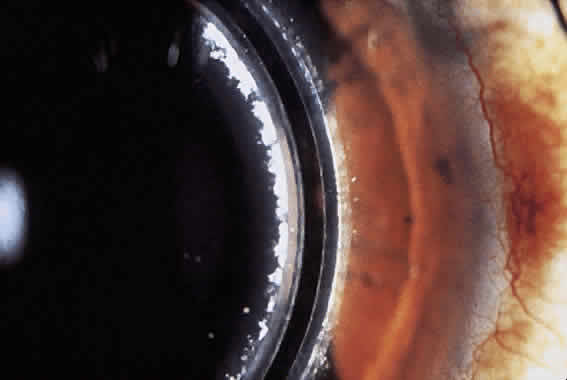
Although microperforations were the most common incisional complication encountered during RK, encroachment of the central clear zone was often the most visually disabling. Patients would complain of significant glare and halos around lights. Also, the resultant irregular astigmatism and corneal opacities often reduced the best spectacle-corrected visual acuity (BSCVA). Occasionally, patients would need corneal transplantation.
The original Russian incisions had a higher risk of inadvertent encroachment of the visual axis. Using this technique, the surgeon was required to terminate the incision manually at the marked clear zone. Unexpected movements by either the surgeon or the patient often caused the blade to cross the visual axis.
Irregular RK incisions are additional sources of subjective halos and glare. All RK incisions are created freehand. Thus, the regularity, linearity, and quality of the incisions are largely surgeon-dependent. Surgeons have created markers for the cornea to guide the incisions radially. However, angulation of the blade can create a beveled incision, reducing depth and thus the effect of the incision.
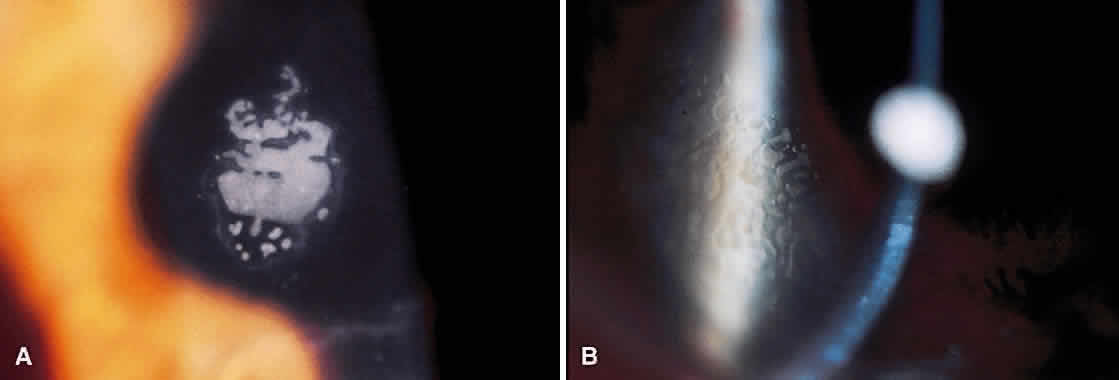
Current techniques have evolved to eliminate most of these complications. The diamond blades used today have two cutting edges. The main cutting edge is sharp for the entire length of the blade, whereas the anterior surface has a cutting surface for only 250 μm (Fig. 1). The blunt portion of the blade helps prevent inadvertent encroachment of the visual axis. Also, the new blades have a housing that will glide along the corneal surface to help keep the incisions perpendicular.
|
POSTOPERATIVE COMPLICATIONS
The advances made in instrument design and surgical technique have greatly limited the incidence of intraoperative complications. However, postoperative complications continue to cause significant problems. Immediately after the procedure, the patient often feels a foreign body sensation for 1 to 3 days because of the breached epithelium. Topical nonsteroidal anti-inflammatory medications (NSAIDs) have been shown to be successful in reducing postoperative pain10 and are widely used following RK, photorefractive keratectomy (PRK), and LASIK. The epithelium is generally healed after 48 to 72 hours, with the resolution of symptoms.
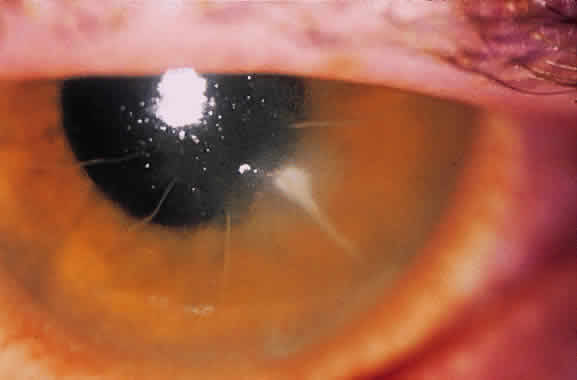
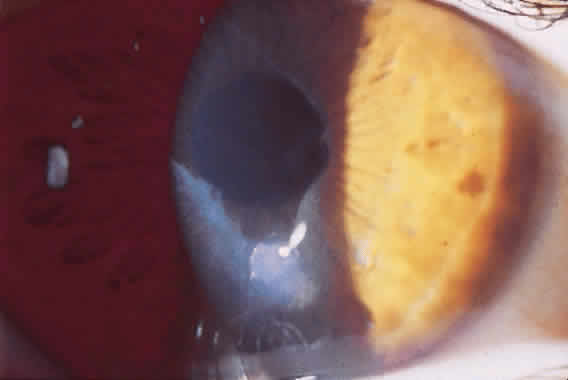
Until the epithelium completely heals, the risk of postoperative infection is greatest. Post-RK bacterial keratitis is primarily the result of the break in epithelial barrier created by the incisions. Adherence of causative organisms to the denuded epithelium over radial incisions can lead to infectious keratitis (Fig. 2). Recently, Panda and colleagues11 highlighted the severity of bacterial keratitis, despite its low incidence. They reported nine cases of post-RK bacterial keratitis at a tertiary-care cornea service during a 9-year period. Four of the nine cases followed primary RK and five followed enhancement procedures. Therapeutic penetrating keratoplasty was performed in one patient. Four of the nine eyes required subsequent penetrating keratoplasty, and one required lamellar keratoplasty. All but one eye retained 20/60 vision or better. These results are discouraging, but the actual incidence of bacterial keratitis lies somewhere between 0% and 0.4%.9,12–14 The PERK study found only 3 cases of bacterial keratitis in 793 eyes (0.38%); all occurred more than 7 months after surgery. All cases occurred in inferior incisions, and only one was spontaneous (one case with contact lens use and another with ocular trauma). None of the three eyes lost BSCVA. Hoffer and associates14 reported only one case of corneal ulcer after RK in 134 procedures performed at Jules Stein.
Post-RK bacterial keratitis usually occurs within 2 weeks of the procedure and is caused by virulent bacteria. The most common causative organisms are listed in Table 1. Infiltrates most commonly arise in the RK incision. Culture and Gram stain should be performed, and frequent fortified topical antibiotics that target the most common organisms should be used until the acute infection is under control. These cases of bacterial keratitis can result in significant vision loss, but if treated appropriately these infections can often be controlled with minimal reduction in vision.11,15 If the site of keratitis coincides with an area of microperforation or macroperforation, aggressive therapy and follow-up are required to avoid bacterial endophthalmitis.16–19
TABLE 1. Etiologic Agents Involved in Infectious Keratitis Following Radial
Keratotomy
Acute keratitis (1 day to 2 weeks)
Staphylococcus aureus
Staphylococcus epidermidis
Stretpcococus pneumoniae
Pseudomonas species
Serratia marcescens
Delayed keratitis (>1 month)
Staphylococcus epidermidis
Pseudomonas aeruginosa
Moraxella
Propionibacterium acnes
Serratia liquefaciens
Enterobacter gergoviae
Atypical keratitis
Candida parapsilosis
Mycobacterium chelonae
Herpes simplex virus
An entity unique to RK is a delayed keratitis, seen up to 40 months after surgery.20,21 The cause is unclear, because each case was associated with positive bacterial cultures. Several cases were associated with contact lens use, inducing microtrauma to the corneal epithelium. Aside from an infectious cause, some theorize that epithelial plugs in RK incisions contain cysts that rupture, leading to sterile inflammation. These cases of sterile keratitis respond to topical corticosteroids. If postoperative keratitis develops, however, therapy should be aimed at infectious causes before the use of topical steroids.
REFRACTIVE COMPLICATIONS
Early results of RK were encouraging: nearly 85% of patients gained uncorrected visual acuity (UCVA) of 20/40 or better at 1 year.8,9 Creation of nomograms for the number and location of incisions allowed more accurate treatments, but overcorrections and undercorrections continued to be a problem. Undercorrection is often a desirable result of refractive surgery, especially in presbyopic patients.
Attempts to treat overcorrection induced by RK were often unsuccessful. However, surgeons now have many options available to treat overcorrection with variable success. Before the use of the excimer laser, laser thermokeratoplasty, keratomileusis, lamellar keratoplasty, and various suture techniques were used. The most popular suture technique is the lasso technique, where one 10-0 nylon suture is passed through the radial incisions at a 7-mm optical zone. Results are initially promising, but some surgeons report regression of effect over time.22 More recently, LASIK has shown promise in reversing overcorrection.23
Undercorrection after RK is easier to correct. Enhancement rates varied from 0.6%7 to 42%,24 with 9% of the PERK patients having an enhancement procedure in the first year.9 An enhancement involved either deepening existing incisions to obtain full refractive effect or creating additional incisions. Despite the large rate of enhancements in some studies, the success rate of enhancements alone has not been addressed in the peer-reviewed literature.
During the long-term follow-up of RK patients, a hyperopic shift was noted in up to 54% of eyes.25,26 The rate of hyperopic shift seen in the PERK study was most pronounced in the first 2 years (+ 0.21 D per year) and continued up to 10 years after surgery (+ 0.06 D per year). When the authors looked at factors that influenced the development of a hyperopic shift, only the size of central clear zone and corneal diameter (and thus the length of incision) were correlated. Smaller clear zones and larger corneal diameters were weakly associated with a greater hyperopic refraction at 10 years. Lindstrom27 addressed this issue with the development of mini-RK, in which the incisions were 3 to 4 mm long. Lindstrom demonstrated that the peripheral extent of the incisions did not significantly affect the amount of central flattening and was able to obtain similar refractive results while theoretically limiting the postoperative hyperopic shift.
The refractive instability was not the only chronic problem: daily fluctuations in visual acuity were not uncommon. Some patients needed to take a nap to restore their vision. In addition, patients reported changes in vision when the barometric pressure changes. Intrigued by this phenomenon, Winkle and associates28 demonstrated that the hyperopic shift seen at high altitude was a result of corneal hypoxia, not the alteration of barometric pressure.
ANATOMIC COMPLICATIONS
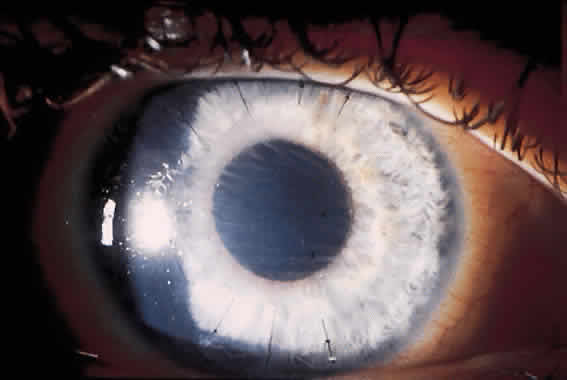
Along with refractive instability, 90% corneal thickness incisions decrease the integrity of the cornea. Patients with RK incisions are at greater risk of serious eye injury from trauma. Histologic analysis of the healing corneal incisions reveals that healing is not completed for more than 5 years after RK.29 These healing wounds may rupture from direct ocular trauma or during intraocular surgery.30 Even after the healing process is completed, the structural integrity of the cornea remains diminished over its preoperative state.31 Other structural abnormalities were discovered after RK. Nelson and colleagues32 described map-dot-fingerprint changes in the corneal epithelium after RK. These patients did not develop recurrent erosions, but the changes were visually significant.
Controversy still exists as to the effect of RK on the corneal endothelium. A recent report states that although there is an acute loss of endothelial cells, the total rate of cell loss was not significant after 7 years compared with contact lens-wearing controls.33 However, there have been several studies demonstrating a significant loss of endothelial cells and resultant loss of function.34–36 With the incidence of microperforations nearing 40% in some studies, it is likely that many incisions approach Descemet's membrane. This may directly alter the interaction of the corneal endothelium and overlying stroma.
LOSS OF VISUAL ACUITY
The most crucial statistic reported for refractive surgery is loss of BSCVA. The PERK study reported that only 3% of patients lost more than two lines of BSCVA at 10 years.25 Arrowsmith and Marks12 found that nearly 15% of patients had a similar loss; however, only two of their patients had postoperative BSCVA of worse than 20/40. From this standpoint, RK was a safe procedure.
CONCLUSIONS
Complications of RK are well reported in the medical literature because it was the first popular procedure to correct myopia. Subsequently, the ophthalmologic community demanded controlled studies to evaluate its success and complications. Just as with Sato's original posterior incisional keratotomy, the complications associated with the procedure did not become evident until the long-term studies were published. Thus, the popularity of RK has diminished to give way to more predictable, safer surgeries. The refractive and structural instability has kept ophthalmologists from recommending RK except in certain circumstances. RK still has a place in the refractive surgeon's armamentarium, but it is now reserved for very low myopes and patients who are not candidates for the laser refractive procedures.
Many surgeons continue to perform incisional keratotomy to correct corneal astigmatism. Whether performed during cataract surgery or as an adjunct to laser refractive surgery, astigmatic keratotomy (AK) carries the same risks as RK. Infectious keratitis, corneal perforation, and refractive fluctuations can complicate even minor astigmatic correction. Therefore, strict sterility precautions and guarded diamond blades must be used with astigmatic keratectomy just as with RK.