ROLE OF THE CORNEAL AND CONJUNCTIVAL EPITHELIUM
The corneal and conjunctival epithelia merge with each other at the limbus, where the subtle transition from the nonkeratinized, stratified squamous epithelium of the cornea to the nonkeratinized, stratified columnar epithelium of the conjunctiva occurs. These two epithelial surfaces together cover the exposed portions of the eye and function as a barrier to chemical insults and to invasion of microorganisms.
The Corneal Epithelium
The corneal epithelium serves the unique function of providing a smooth optical surface and the transparency necessary to transmit images with minimal distortion. The chemically-injured corneal epithelium may desquamate, or it may become irregular and lose its clarity. When the integrity of the epithelium is compromised, exposure of the underlying stroma may result in an alteration of hydration that further compromises corneal transparency.
The epithelial cell membranes are composed of lipid, which renders these cells hydrophobic. Adjacent cells are linked by secure junctional complexes, which impede the penetration of caustic substances through the epithelium. Strong alkalies pass rapidly through this relative barrier, however, because they saponify and liquefy the lipoidal cell membranes and junctional complexes.
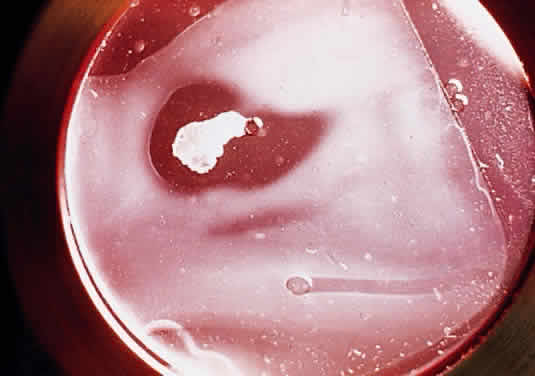
Epithelial cells can be vigorous contributors to various phases of the corneal immune response.14,15 Intermingled with corneal epithelial cells are sparse Langerhans cells,16,17 which appear during local or remote corneal inflammation18 and which also participate in the corneal immune reaction.19 Epithelial cells secrete cytokines, including those that can inhibit the production of type I collagenase (capable of digesting corneal stroma) by underlying keratocytes.20 Curiously, epithelial cytokines can also stimulate the same keratocytes to produce type I collagenase.21 In stromal ulcerative disorders, for example after severe chemical injuries, regulatory participation of regenerating epithelium can help to tip the balance toward either further stromal degradation or re-establishment of corneal integrity. Epithelial cells themselves can produce a collagenase, although it is type V collagenase (gelatinase), which digests a substrate of denatured collagen (Fig. 1).22 Epithelial cells also can release prostaglandins in response to inflammation.14
|
Source of Regenerating Corneal Epithelium
The undisturbed corneal epithelium consists of approximately five layers of cells overlying the basal epithelial cells, which are affixed to their underlying basement membrane (Bowman's layer) through a group of adhesional components, primarily the hemidesmosomal anchoring plaques. The presence of an intact extracellular matrix, with fibronectin, laminin, glycosaminoglycans, and collagen is essential for secure binding of the basal epithelial cells.23
Epithelial cells arising from multipotential stem cells at the corneoscleral limbus migrate continuously in a centripetal fashion toward the corneal center. They replace the epithelial cells that have moved toward the surface during their normal maturation and have desquamated from the cornea.24 In the uninjured cornea, complete replacement of epithelial cells occurs every 5 to 7 days.25 The rate at which migrating cells move over the corneal surface increases after a traumatic loss of corneal cells,26 for example after chemical injuries resulting in epithelial desquamation. In the ideal situation, sheets of advancing corneal epithelium impinge on the epithelial defect in convex waves that eventually meet in healing ridges resembling the arms of a “Y”. These ridges later disappear as the healing cells readjust their positions to restore the normal contour of the injured cornea.
Within the first few hours after corneal epithelial injury, the surviving margin of intact epithelium sends fingerlike extensions forward into the injured zone. Fibronectin and other proteins from the tear film are deposited on the bare stroma or intact Bowman's layer. By the sixth hour, basal epithelial cells from the margin of the wound have lost their hemidesmosomal attachments and have become mobile. They migrate centripetally into the denuded zone, dragging with them one or two overlying layers of epithelial cells. At first, individual cells become thin, increasing their surface area to facilitate migration over the defect. Later, their numbers increase as mitosis occurs a few millimeters behind the advancing edge. Only after the defect is completely closed do the healing epithelial cells establish secure attachments to the underlying basement membrane and extracellular matrix. Next, they synthesize the proteins and intercellular bridges that render an intact epithelial surface resistant to penetration by infectious agents and noxious chemicals.25–27
The Conjunctival Epithelium
The conjunctiva is a vascularized mucous membrane that lines the entire exposed surface of the eye posterior to the cornea. It is reflected through the conjunctival fornices onto the posterior aspect of the lids. Its epithelium provides a moist, smooth surface over which the lids can pass. It participates with the corneal epithelium in establishing a relative barrier to the passage of microorganisms and noxious chemical agents, and it is active in local immune reactions.28 Its goblet cells produce mucin, which adsorbs to the glycoproteins coating the microvilli of corneal and conjunctival epithelial cells. This precorneal and preconjunctival mucin layer merges gradually with the overlying aqueous tear film to ensure complete wetting of the ocular surface.
The conjunctival epithelium provides a source of cells to repopulate the corneal surface when the entire corneal epithelium has been denuded and the limbal stem cells have been destroyed, as in severe chemical injuries.29,30 After complete re-epithelialization of the cornea with conjunctival epithelium, a phenotypic change in the structure of these cells takes place by the process of transdifferentiation.31 Gradually the new epithelial surface begins to resemble that of corneal epithelium. The pseudostratified, columnar conjunctival epithelium becomes pseudostratified squamous, and there is an attrition of goblet cells. After several weeks, the biochemical functions of the healing cells begin to resemble more closely those of corneal epithelium. After transdifferentiation in cases of severe chemical injury, however, vascularization of the healing tissue inevitably occurs, with a return of goblet cells and conjunctivalization of the corneal surface.32
ROLE OF THE CORNEAL STROMA
Ninety percent of the cornea is stroma, consisting of approximately 200 layers of mostly type I collagen.33 Resting on the most superficial portion of the stroma is Bowman's layer, a meshwork of filaments derived from collagen types I, III, V, VI and possibly IV.34 The stroma itself is relatively acellular, with only 2% occupied by keratocytes, which are the corneal equivalent of fibroblasts. These keratocytes produce collagen, which accounts for more than 70% of the stroma by weight. The keratocytes secrete into the extracellular space a procollagen triple helix, from which collagen molecules form. These coalesce into fibrils, which in turn assemble into collagen fibers. Collagen fibers are uniform in diameter, with a constant spacing, factors which favor the transparency of corneal stroma.35
Keratocytes also synthesize glycosaminoglycans, which help to regulate water metabolism in the stroma as well as maintain the stroma's transparency. Most of the water in the intercellular stroma is bound to glycosaminoglycan molecules, which maintain uniformity of collagen fibril spacing and the cornea's transparency.35
Keratocytes also fabricate matrix metalloproteinases (MMPs),36 otherwise collectively known as matrixins or collagenases. These proteases regulate the synthesis and degradation of extracellular matrices, and participate vigorously in corneal wound healing. The MMPs consist of nine types, of which MMP-1 and MMP-8 are true collagenases, MMP-2 and MMP-9 are gelatinases (which utilize a substrate of degraded collagen), and the remaining varieties (MMPs 3, 7, 10, 11, and 12) are stromelysins.36 MMPs are critical components of the process by which injured extracellular matrix is digested and later reformed. MMPs are regulated in vivo by tissue inhibitors of metalloproteinases (TIMPs) and other inhibitors.36 Imbalance between MMPs and TIMPs can lead to excessive fibrosis (when the inhibitor exceeds the enzyme) or to excessive tissue melting (when the enzyme exceeds the inhibitor).36 The major proteinase inhibitors of the extracellular matrix are α2-macroglobulin,36 which also constitutes most of the anticollagenase activity in plasma,37 and the TIMPs.
Among the other participants in corneal wound healing are the various growth factors, including epidermal growth factor (EGF) and transforming growth factors (TGFs) α and β. These are mitogens and chemotactic agents.38 In cornea, there is a constant interaction between epithelium and stroma in the repair of stromal injury.39 For example, TGF beta-2 from epithelial cells in culture inhibits collagenase synthesis by cultured corneal stromal cells; other epithelial cytokines stimulate stromal keratocytes to produce collagenase.39
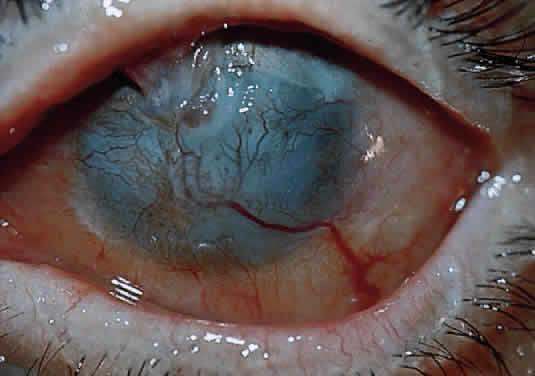
After a chemical burn or similar injury, keratocytes increase in number by mitosis, and new ones migrate into the region of damage. Migration of keratocytes occurs only under an intact epithelium.34 EGF and fibroblast growth factors both help to regulate the influx of new keratocytes.40 The energized keratocytes produce new collagen and proteoglycans.34 Although the new collagen is type I,41 the diameter of the resulting fibers is larger and the spacing is irregular,42 decreasing transmission of light through the resulting scar. Meanwhile, the new proteoglycans bind water more avidly, resulting in excess hydration of the scar, which further insures irregular spacing (with lack of transparency) of the new collagen.34 In addition, stromal keratocytes develop intracytoplasmic contractile elements that cause contraction of the scar and irregular astigmatism.34
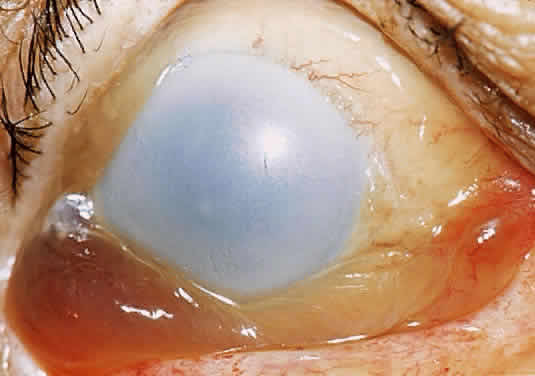
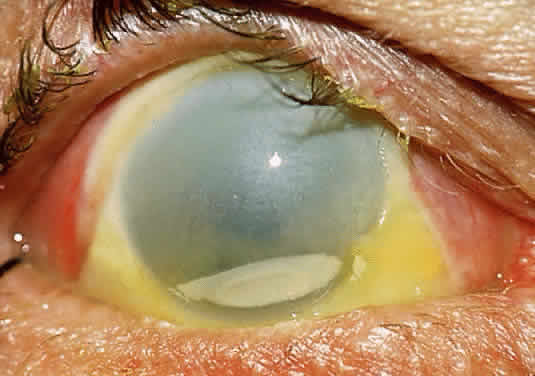
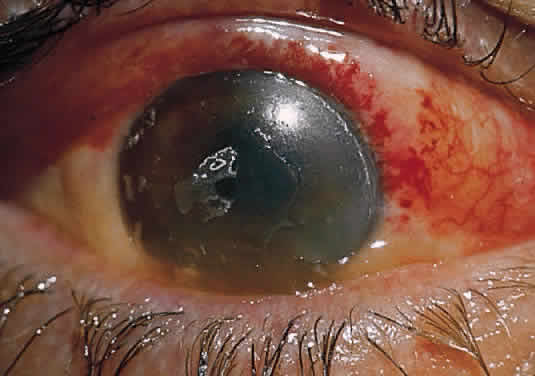
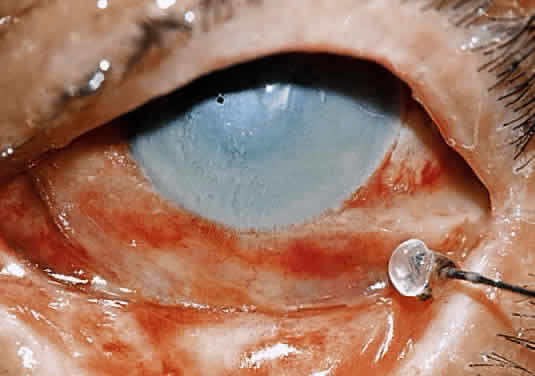
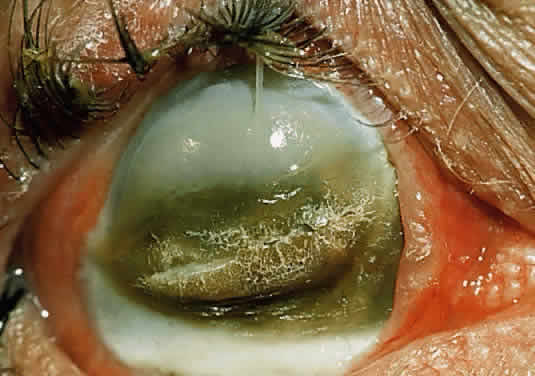
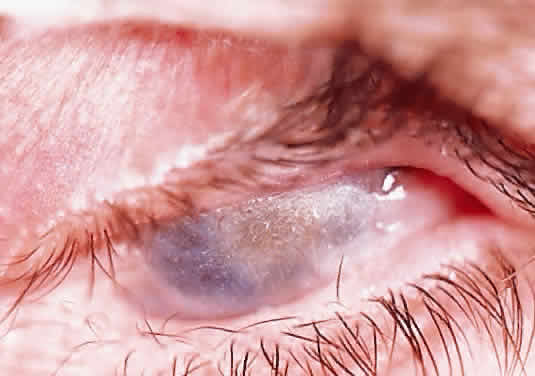
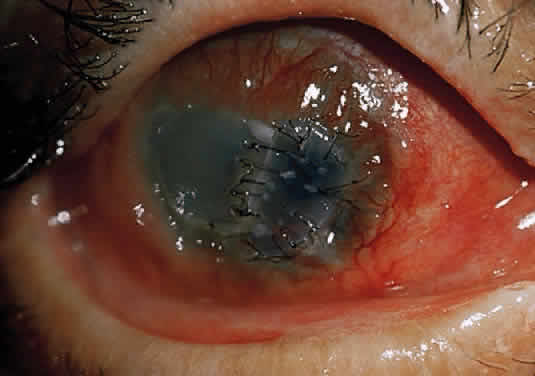
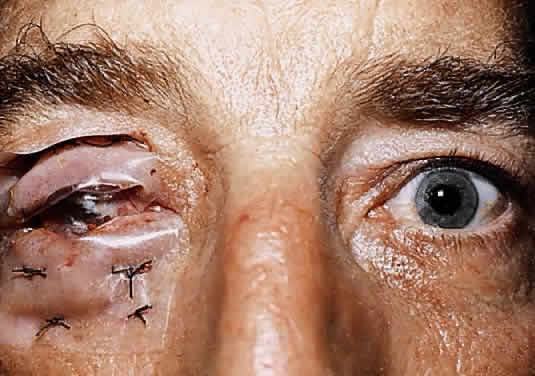
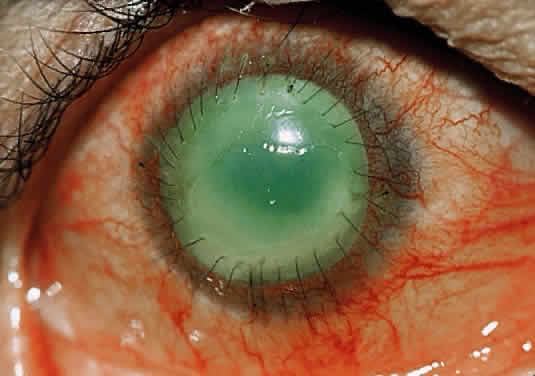
In the chemically-injured cornea, these complex mechanisms by which the stroma responds typically lead to minimal scarring and loss of transparency when an overlying epithelial defect heals rapidly and the damage to the stroma is superficial. In injuries of great severity, with long chemical contact time and maximal variances from physiological pH, the overwhelming stromal response is that of degradation or melting. These are situations in which the intrinsic control mechanisms fail to maintain homeostasis.
ROLE OF THE POLYMORPHONUCLEAR NEUTROPHIL
Collagenase activity has been documented in polymorphonuclear neutrophils (PMNs).43 Before they are released from the bone marrow, PMNs synthesize proteases, including gelatinase B and collagenase 2.44,45 Matsuda and Smelser observed numerous PMNs at sites of stromal ulceration after ocular chemical injuries.46 Pfister and colleagues observed that after alkali injury of the cornea, the tripeptide degradation products of stromal collagen are among the earliest biochemical factors stimulating PMN influx into the cornea.47,48 The gathering PMNs themselves release leukotrienes, which are chemotactic agents for the additional influx of neutrophils.49 The alkali-injured collagen also liberates a cytokine that triggers a respiratory burst among the accumulating PMNs. These cells release not only proteolytic enzymes, but also superoxide radicals, both of which aid in further collagen degradation and resultant corneal ulceration.49
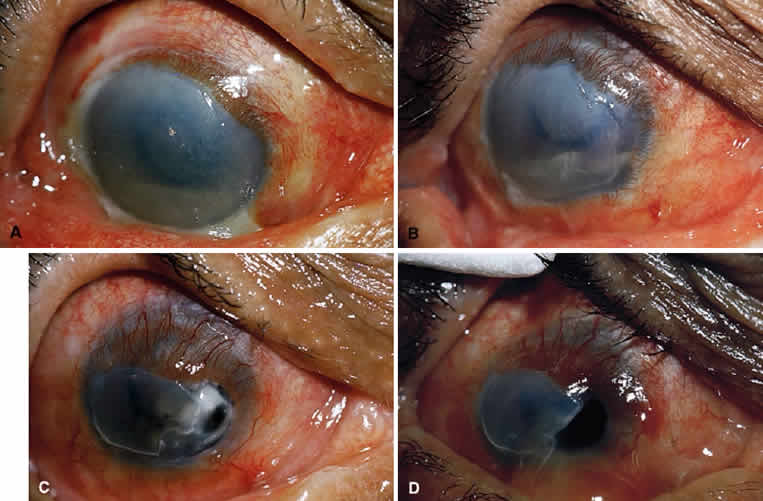
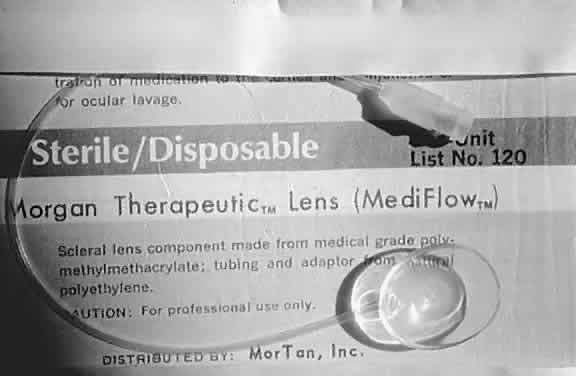
Kenyon and colleagues demonstrated that in rabbit eyes, after burns with sodium hydroxide, when both epithelium and PMNs were excluded from the exposed stroma by gluing a contact lens in place two days after the burn, only 10% of corneas ulcerated.50 In the control eyes without corneal protection, ulceration occurred in 2 weeks. If the lenses were removed at day 14, 80% of corneas ulcerated within the next week. When rings (exposing the corneas centrally) were glued onto the denuded stroma of 15 burned eyes, epithelium was excluded but PMNs migrated to the exposed central zone through the tear film. Eleven of the corneas developed ulcerations, with PMNs adhering to the digested tissue. In non-alkali-burned controls, with or without glued-on contact lenses or rings, no ulcerations occurred despite the presence of many PMNs.50
Evidence suggests that 10% sodium citrate applied hourly to the alkali-burned eye may offer some relative protection against stromal ulceration51 by inhibition of selective PMN activities. The PMNs must adhere to the endothelium of vessels before their movement through the vascular wall and chemotaxis can cause a tissue infiltrate. Citrate inhibition of PMNs may be through a mechanism that keeps them circulating and prevents them from adhering to the endothelium.52