PHYSICAL FACTORS
Epithelium
The corneal epithelium is composed of five to seven cell layers with a thickness of approximately 50 μm, accounting for about 10% of the total corneal thickness. The cornea is covered by nonkeratinized, stratified squamous epithelium that is constantly sloughed and regenerated. Limbal stem cells produce epithelial cells that migrate centrally and superficially. The epithelium consists of flat superficial cells, deeper winged cells, and an underlying monolayer of columnar basal cells. The basal cells are responsible for secretion of a basement membrane adjacent to Bowman's layer, which maintains organization of the epithelium and acts as a scaffold on which cells can migrate. One important function of the epithelium is to act as a mechanical barrier to corneal absorption of fluid from tears that may contain instilled topical medications or pathogens residing on the ocular surface. Tight junctions are present in the superficial epithelial cells that help serve a barrier function. Electrical resistance of the cornea predominantly resides in the epithelium, and the resulting impermeability of the epithelium is important in the pharmacokinetics of topical medication penetration.3 The endothelium and stroma have a lower electrical resistance, leading to greater permeability of these layers.4
The transparency of the normal epithelium is the result of the homogeneity of the refractive index of cells throughout this cellular layer.5 When epithelial edema occurs, the epithelium loses its homogeneity and the corneal surface becomes irregular. This surface irregularity causes a reduction in vision along with symptoms of glare, photophobia, and halos around light due to light scatter. The effect of epithelial edema on vision depends on ambient lighting conditions. In mesopic conditions, such as the ophthalmologist's examining room, there may be minimal effect on visual acuity. However, in bright light, edematous epithelium creates light scatter, with marked vision. Surface irregularities caused by epithelial edema are more damaging to vision than stromal edema or scarring. Often the influence of the epithelial surface irregularities on visual acuity is underestimated, whereas the role of stromal scarring and stromal edema is overestimated.
Stroma
The stroma makes up approximately 90% of the corneal thickness and has a composition of uniformly arranged collagen fibrils in lamellae. The collagen fibrils are surrounded by a ground substance made up primarily of glycosaminoglycans, which include keratan sulfate, dermatan sulfate, and chondroitin. The stroma is for the most part an extracellular compartment with keratocytes and nerves accounting for only 5% and 0.01% of its volume, respectively. Water makes up approximately 70% of the stromal volume.
The corneal stroma plays a primary role in maintenance of corneal shape and physical strength. Its constituents help preserve transparency in conjunction with the endothelium. Stromal edema may be caused by malfunction of the endothelium and/or epithelium. When the stroma swells, the diameter of the collagen fibrils remains constant. Swelling is caused by an increase in fluid in the ground substance, leading to an increased anteroposterior spatial separation between the lamellae of collagen fibrils.6 Because the collagen lamellae are oriented in the direction of the corneal diameter, the diameter of the cornea does not increase with corneal edema.
Endothelium
The endothelium is a monolayer of homogeneous, closely packed, polygonal cells approximately 5 μm thick. The endothelial contribution to maintenance of stromal deturgescence and transparency occurs by two mechanisms. The first mechanism is the barrier function of the endothelium between the cornea and aqueous components, a passive barrier that is much less efficient than the epithelial barrier function. The second mechanism involves the pump function of the endothelium through use of active sodium-potassium adenosine triphosphatase (Na-K ATPase) pumps that actively remove fluid that leaks into the stroma from the aqueous compartment.
The normal endothelial cell density is 3000 to 3500 cells/mm2 in the young adult. The number decreases by about two-thirds in elderly patients.7 Even though human corneal endothelial cells have been shown to proliferate in laboratory cell cultures, they have little or no ability for mitosis after birth in vivo.8 When endothelial loss occurs through aging or trauma, the endothelial response is enlargement and sliding of the existing cells to cover the area previously occupied by the lost cells. When cell loss decreases cell density below a critical number (usually 500 cells/mm2, depending on the health of the cells), corneal edema ensues.
CORNEAL HYDRATION
In addition to physical factors, the transparency of the cornea depends on its hydration status. To maintain transparency, the cornea must remain relatively thin and dehydrated. The corneal stroma of most vertebrates, including humans, swells if placed in aqueous solution because the osmotic load of the glycosaminoglycans in the stroma draws fluid into the stroma. Because the cornea swells only in the direction of its thickness, corneal thickness and hydration are linearly related. This linear relation allows measurement of corneal hydration by measurement of corneal thickness. Preservation of adequate corneal dehydration results from the following five factors: stromal swelling pressure (SP), the barrier function of the epithelium and endothelium, the endothelial pump, evaporation from the corneal surface, and the intraocular pressure (IOP). Understanding the mechanisms of corneal hydration is critical in the management of patients with corneal edema.
Stromal Swelling Pressure
An excised cornea is normally 78% hydrated, but hydration can increase up to 98% when the cornea is placed in aqueous media, with a proportional increase in its thickness. The stromal swelling is due to interfibrillary imbibition of water, not swelling of collagen fibrils. If one extracts the glycosaminoglycans from the stroma, a marked reduction in swelling occurs, indicating that glycosaminoglycans are the major cause of this hydration phenomenon.2 Keratan sulfate and chondroitin both have fixed negative charges that repel each other, and electrostatic repulsion between the negative charges appears to be a major force involved in the swelling. Because of the poorly cross-linked configuration of the collagen fibrils, the glycosaminoglycans can expand with very little resistance. This unhindered expansion results in the marked degree to which the stroma can swell.
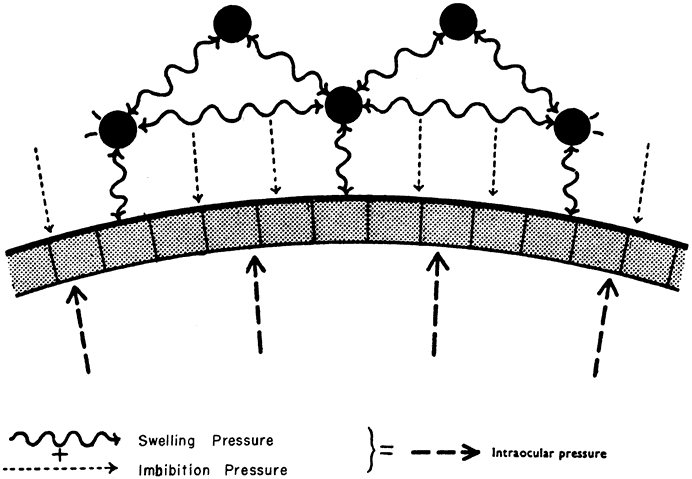
The stroma is normally maintained in a relatively dehydrated state compared with its ability to swell. The potential ability of the stroma to swell decreases as its hydration increases. The SP of the normal excised cornea is 50 mm Hg. SP is caused by the presumed anionic repulsion of the glycosaminoglycans, which expands the tissue and draws fluid into the stroma. The negative pressure drawing fluid into the cornea is called imbibition pressure (IP). In excised corneal tissue, the values of SP and IP are equal. However, in vivo, IP is lower than SP because of the compressive effect of IOP on the stroma. The following relationship establishes imbibition pressure: IP = IOP – SP. The compressing effect of IOP on the endothelium in vivo creates an IP of 30 to 40 mm Hg (Fig. 1). Water flow occurs from inside the eye as aqueous percolates into the corneal stroma and evaporation occurs from the epithelial side.
Glycosaminoglycan molecules create a high resistance to water flow across the stroma in the normally dehydrated cornea. The resistance is reduced as corneal hydration increases, so that in the more edematous cornea, there is less resistance to increased water flow across the stroma. There is no lateral flow of water in the cornea except at the limbus.9
Barrier Function of Epithelium and Endothelium
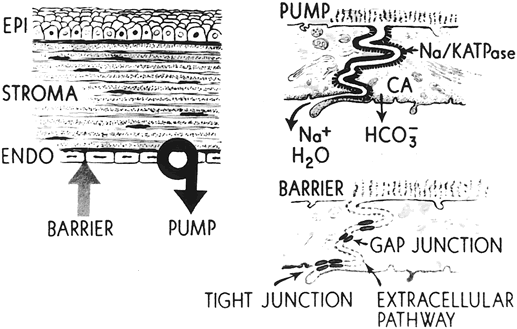
Both the epithelium and endothelium act as semipermeable membranes, creating a barrier to diffusion of electrolytes and to the flow (not diffusion) of water. The epithelium offers twice the resistance to flow of water than the endothelium, and relative resistance to diffusion of electrolytes is 200 times greater in the epithelium than in the endothelium.10 Therefore, the epithelium constitutes a relatively impermeable membrane. The ultrastructural basis for this impermeability comes from the tight junctions known as zonula occludens that seal the intercellular space between the superficial epithelial cells. By contrast, gap junctions bridge the intercellular space at the apical end of the endothelial cells.11 Permeation of small ions and water into the stroma from the aqueous is due to the semipermeable nature of the endothelium and is driven by the IOP and negative IP of the stroma. The interaction of all these forces on the hydration of the corneal stroma is shown in Figure 2. Factors that affect barrier function are listed in Figure 3.
|
Endothelial Pump
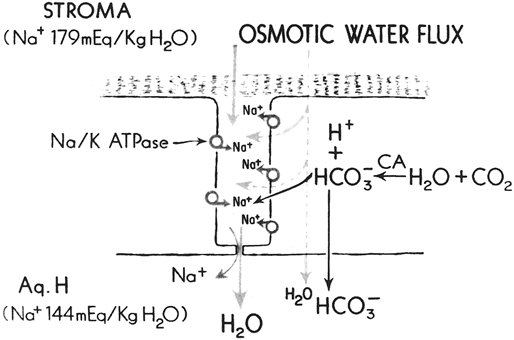
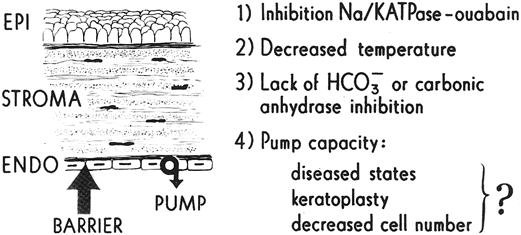
A constant amount of fluid leakage occurs across the endothelium from the anterior chamber, a finding that validates the fact that another mechanism aids in maintaining corneal deturgescence. The cornea has been observed to thicken in cooled enucleated eyes, whereas subsequent warming promotes thinning to the original thickness.12 This “temperature reversal” of hydration requires oxygen and does not occur in the presence of metabolic inhibitors, such as ouabain, which block cation transport.13,14 Deturgescence with warming suggests that there is an active transport of water and/or electrolytes from the stroma in order to maintain normal corneal hydration. Furthermore, after the epithelium has been removed, the temperature reversal effect still occurs, showing that the metabolic pumps exist within the endothelium.13 Evidence now points to ion transporter complexes within the endothelium that facilitate the passive secondary movement of water inflow.
Although the active transport of sodium and potassium ions may play a role in the endothelial pump, the major component of the pump is the active transport of bicarbonate ions into the aqueous humor.15 A negative electrical potential exists on the aqueous side of the endothelium, suggesting the bicarbonate anion, rather than the sodium or potassium cation, is responsible for the fluid transport across the endothelium.16 The endothelial pump is dependent on oxygen, glucose, carbohydrate metabolism, and ATPase (Fig. 4). Dactinomycin, ouabain, and oligomycin are potent inhibitors of this system. Factors that influence the endothelial pump function are listed in Figure 5.
|
|
Evaporation From the Corneal Surface
Evaporation was shown to play a major role in the thinning of rabbit cornea.17 However, only 4% of thinning occurs in humans as a result of osmotic extraction of fluid due to tears made hypertonic by evaporation.18 This loss of fluid is readily replaced by aqueous and therefore plays little role in corneal dehydration.
Intraocular Pressure
Elevated IOP can cause epithelial edema, but it is not associated with a change in corneal thickness. Ytteborg and Dohlman19,20 showed that when the IP becomes positive—that is, when the IOP exceeds the SP—epithelial edema ensues. Microcystic changes may occur in several settings. One case involves high IOP and normal stromal SP and thickness, as in glaucoma. The other setting occurs with a normal IOP and low stromal SP, such as that seen in the corneal edema of endothelial dystrophies. Stromal edema may follow microcystic epithelial changes.
In summary, maintenance of normal corneal hydration is achieved by the barrier function of both the epithelium and endothelium, preventing excessive imbibition of water from the tears and aqueous. In addition, the active transport of some anions and cations contributes to the normal corneal thickness with obligatory transport of water out of the cornea via the endothelial pump.