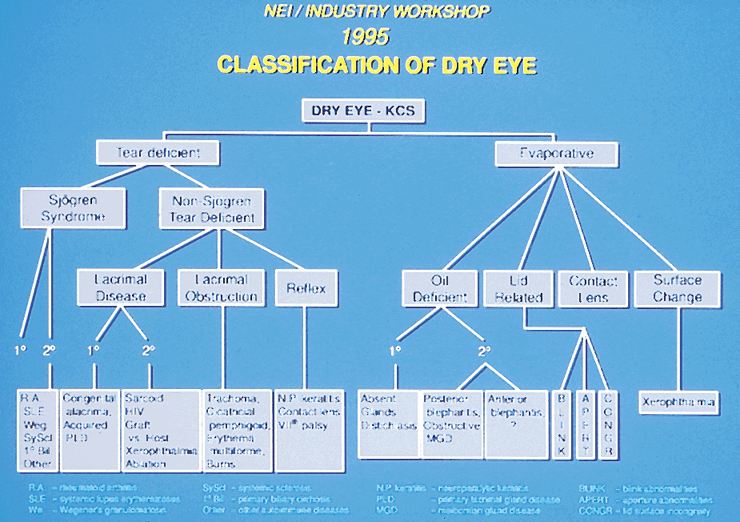
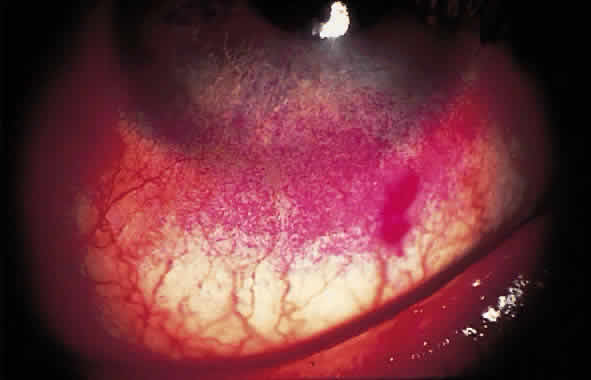
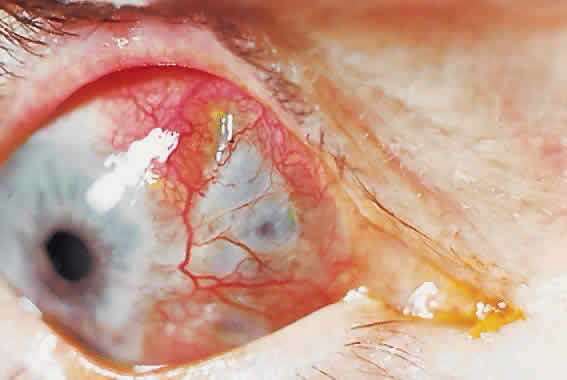
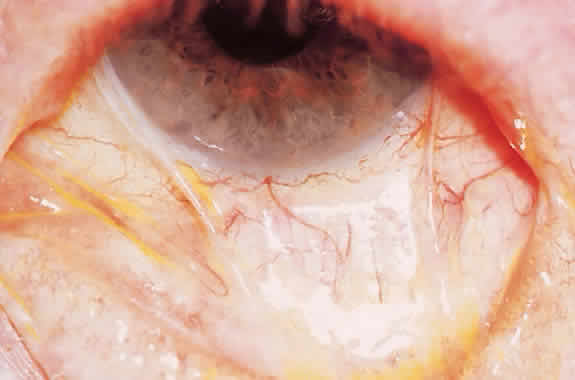
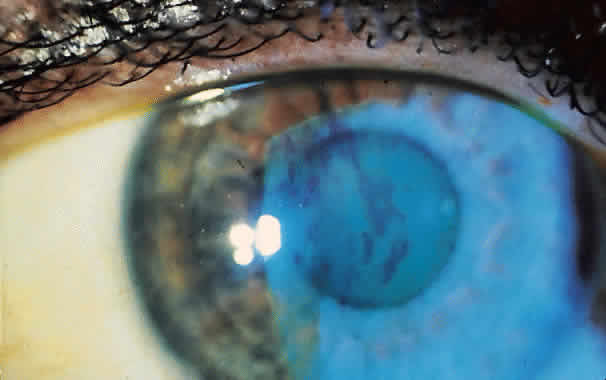
Dry eye is a common disorder affecting a significant percentage of the population, particularly those older than 40 years, throughout much of the world. The prevalence is not well documented throughout the age spectrum but is thought to increase with aging. One type of dry eye, Sjögren's syndrome-associated dry eye, is thought to affect 1% to 2% of the population.1 Evaporative dry eye, primarily meibomian gland dysfunction, is an extremely common type of dry eye and was a factor in up to two thirds of patients who had ocular irritation in a clinical setting.2 Dry eye-associated ocular surface disease is one of the major reasons patients visit ophthalmologists.3
During the past three decades, there has been a remarkable increase in knowledge about the processes that cover normal tear secretion and maintenance of the ocular surface, particularly the pathophysiologic processes that affect these two intimately connected ocular components. Thinking about the pathogenesis of dry eye has shifted slightly from the concept that it is simply a deficiency in the volume of aqueous tears secreted by the main and accessory lacrimal glands.4 It is now known that there are qualitative changes in tear production, particularly in conditions associated with chronic ocular surface inflammation (e.g., vitamin A deficiency, ocular pemphigoid, and Stevens-Johnson syndrome), leading to a dropout of the mucin-producing goblet cells of the conjunctiva.5 This loosely adherent mucin blanket consisting of hydrated glycoproteins plays a role in maintaining the hydration of the ocular surface and contributes to the stability of the tear film. In addition, the epithelial cells of the conjunctival surface produce a mucin-like glycoprotein that adds to the complexity of the role of mucin in the tear film.6
Tears play an important role in maintaining the normal health of the ocular surface and in the repair of injury to the ocular surface. Tears have a role in the regulation of hydration of the cornea by way of tonicity changes secondary to evaporation from the tear film; an osmotic gradient develops across the cornea because of evaporation from the tear film, resulting in movement of fluid from the aqueous to the cornea and to the tear film.
Tears provide the corneal epithelium with its primary source of oxygen. Oxygen is obtained from the atmosphere and dissolved in the tears to support the aerobic metabolism of the corneal epithelium. A small amount of oxygen may be supplied to the peripheral cornea via the limbal circulation. In addition, tears act as a lubricant, facilitating the smooth interaction between the upper lid and the ocular surface. Tears contain several water-soluble substances with antibacterial properties. These include lysozyme, beta-lysine, and lactoferrin. The concentration of these substances in aqueous tears has been used as a marker for tear production.
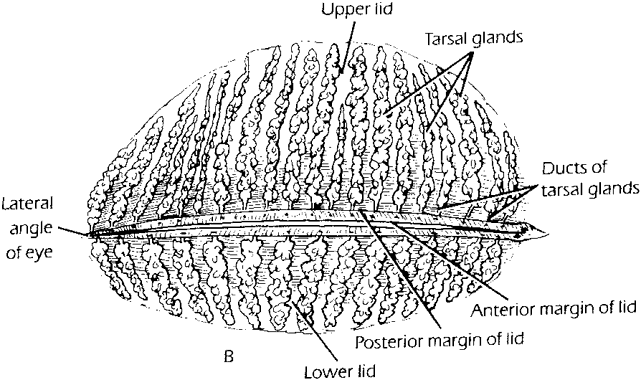
The movement of tears over the ocular surface serves to flush the surface of the eye, removing exfoliated cells, debris, and foreign bodies. This material is further entrapped in a mucin network on the ocular surface7 (Fig. 1). There has been general agreement that the tear film consists of a three-layer structure, with two layers resting on a semisolid mucin layer. The tear film thickness is approximately 7 to 10 μm. The outer layer is composed of lipid and originates primarily from the meibomian glands of the lids (Fig. 2); the glands of Zeis and Moll also contribute. The outer lipid layer of the precorneal tear film can be noted by microscopic observation of the interference patterns seen on the surface of the tear film. The thickness of this lipid layer varies considerably, depending on the interpalpebral area. As the space between the upper and lower lid is narrowed, the lipid layer is compressed. It has been reported to vary from 800 to more than 2000 nm in thickness. The lipid layer retards evaporation from the tear film, prevents contamination of the tear film by more polar liquids secreted by the sebaceous glands of the eyelids, and serves to thicken the tear film by drawing water across the ocular surface. The aqueous layer is 6 to 10 μm thick and constitutes some 90% of the preocular tear film. It is thought that the bulk of aqueous tears arise from the orbital and palpebral portions of the main lacrimal gland. The accessory lacrimal glands, varying considerably in number and weight, are scattered near the upper fornix and open on the conjunctival surface. Their contribution to tear volume varies greatly from one person to another. In addition, there is transconjunctival fluid transport; the contribution of transconjunctival fluid secretion to tear volume is unknown, but some researchers believe it approximates 10% of total volume.
|
|
The aqueous portion of the tears is secreted as an isotonic or slightly hypotonic solution. Aqueous tears flow out of the ductule openings of the main accessory lacrimal glands in the superior fornix. This fluid flows into the forniceal spaces and from there into the lacrimal rivers along the lid margins and over the exposed portions of the cornea and conjunctival surface. The direction of flow is toward the medial canthus. Tear flow is driven by the action of the orbicularis oculus muscle. Some aqueous fluid is lost through evaporation and reabsorption through the conjunctival surface, but most of the fluid flows out through the punctal openings into the superior and inferior canaliculi and then into the common canaliculus and down through the nasolacrimal duct. There is considerable reabsorption of fluid across the mucosa of the nasolacrimal duct during its passage. Total tear volume under relatively nonstimulated conditions is approximately 6 to 8 μl. The flow of aqueous tears has been reported to be about 1.2 μl/min. The previous distinction between basal and reflex tear secretion has been called into question.8 It is probable that all aqueous tear secretion is stimulus-driven. The main and accessory lacrimal glands have a similar histologic structure and are capable of rapidly increasing tear production in response to emotional and physical stimuli.
The aqueous layer contains the water-soluble contents of the tear film. These include inorganic salts, glucose, urea, proteins, and trace elements, all of which are secreted by the main and accessory lacrimal glands. Proteins secreted by the lacrimal glands include lysozyme, lactoferrin, free albumin, lactoperoxidase, transferrin, lipocalin, phospholipase, free albumin, and secretory IgA. Other compounds normally secreted by the lacrimal gland include epidermal growth factor, endothelin-1, basic fibroblastic growth factor, transforming growth factor α, transforming growth factor β, hepatocyte growth factor, thyroid hormone, and retinols.9,10
Lacrimal gland tissue has parasympathetic, sympathetic, and peptidergic innervation. Various substances act as neurostimulators and modulators. These include norepinephrine, acetylcholine, α-melanocyte-stimulating hormone, adrenocorticotropin, and corticotropin-like intermediate lobe peptide. Recently receptors for androgens have been identified in human lacrimal gland tissue in addition to meibomian gland tissue. This and other evidence suggests an important role for locally available bioandrogens in maintaining the normal glandular activity of both the lacrimal and meibomian glands.11
The inner loose mucin layer rests on the underlying surface of the epithelium. Mucin consists of hydrated glycoproteins. The thickness of this layer has been the subject of controversy, but it is probable that this loose mucin blanket extends well into the aqueous layer, contributing to the stability of the tear film. In addition, mucin may serve to affect the shear forces of the upper lid on the ocular surface.
The structure of the tear film is inherently unstable, and its dynamics are the result of a complex interaction between the ocular surface, the tear film itself, and the lids. The bulk of the tear volume is located in the upper and lower tear film menisci. On blinking, the superficial lipid layer is compressed to a thickness of approximately 0.1 mm adjacent to the junction of the closed lids. The underlying aqueous layer remains continuous under the closed eyelids and acts as a lubricant between the lids and the ocular surface. On opening the lids after a blink, the compressed lipid layer is spread in a monomolecular layer over the aqueous tear surface. Most of this spread occurs within 1 second of opening the eye. Between blinks, the tear film is constantly tending toward break-up secondary to evaporation and fluid retraction into the fornices. Periodic blinking resurfaces new tear film and prevents the tendency toward tear film break-up.
The maintenance of normal tear film and, indeed, the ocular surface is dependent on a critical servomechanism involving innervation of the ocular surface, lacrimal glands, and lids. These nervous innervations process signals from the ocular surface that modulate the secretion of hormones, growth factors, retinoids, and cytokines produced by both the lacrimal glands and the ocular surface. These substances in turn direct cellular turnover in the maintenance of the normal ocular surface and repair of injury to the ocular surface. In addition to the critical role of androgens in maintaining the normal glandular function of the lacrimal and meibomian glands, recent evidence points to an important role of apoptosis in maintaining normal lymphocyte populations in the lacrimal glands and in preventing inflammatory disease of the lacrimal glands. Further, dysfunction of normal apoptotic activity in the lacrimal acinar cells and the lacrimal lymphocytes has been suggested as a possible mechanism in the pathogenesis in lacrimal secretion deficiency.12