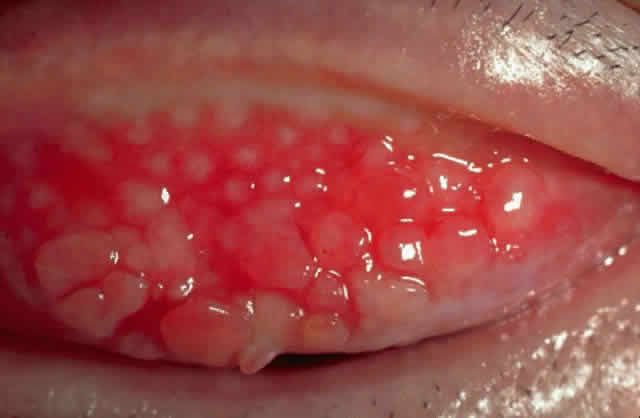
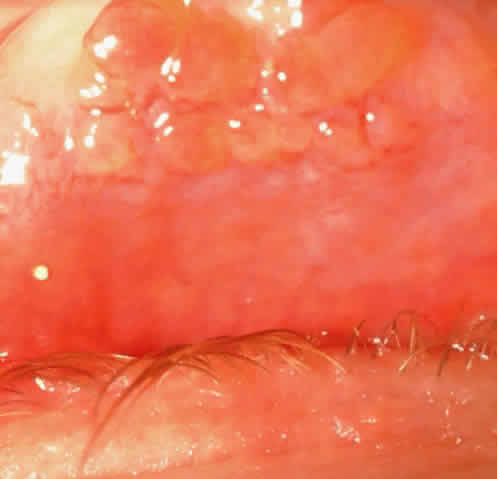
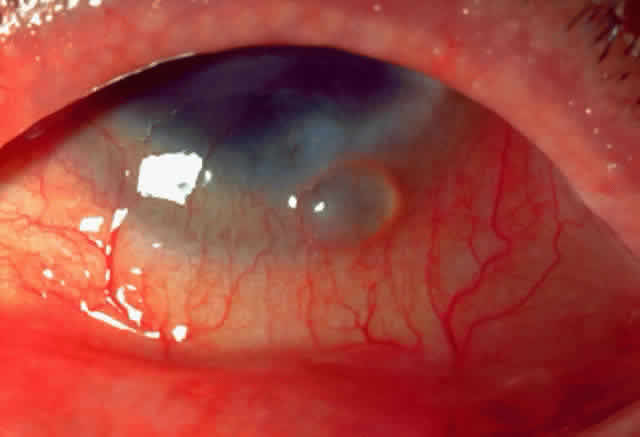
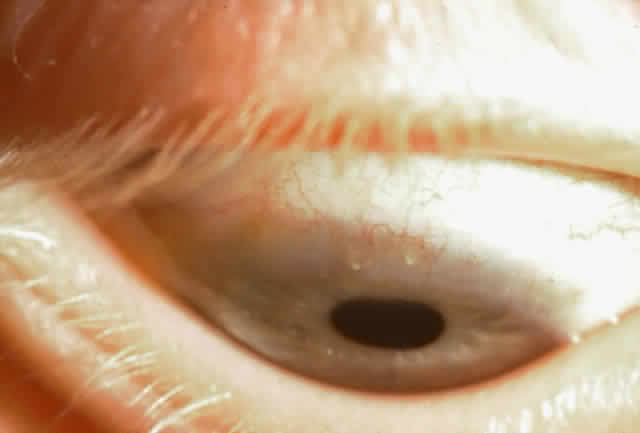
1. Neumann E, Gutmann MJ, Blumenkrantz N et al: A review of four hundred cases of vernal conjunctivitis. Am J Ophthalmol 147:166, 1959 2. Duke-Elder S: Diseases of the outer eye, Part I. In Duke-Elder S (ed): System
of Ophthalmology, p 476. London, Henry Kimpton, 1965 3. Tuft SJ, Dart JK, Kemeny M: Limbal vernal keratoconjunctivitis: Clinical characteristics and immunoglobulin
E expression compared with palpebral vernal. Eye 3:420, 1989 4. Donshik PC, Williams HE: Ocular allergy. In Smolin G, Thoft R (eds): The
Cornea, p 355. Boston, Little, Brown, 1994 5. Jones B: Vernal keratitis. Trans Ophthalmol Soc UK 81:215, 1961 6. Jones B: The differential diagnosis of punctate keratitis. Trans Ophthalmol Soc UK 80:665, 1960 7. Arffa RC: Immunologic disorders. In Arffa RC (ed): Grayson's Diseases
of the Cornea. St. Louis, CV Mosby, 1991 8. Kerr N, Stern GA: Bacterial keratitis associated with vernal keratoconjunctivitis. Cornea 11:355, 1992 9. Stock EL: Immunological mechanisms of allergic disease. Int Ophthalmol Clin 29:262, 1988 10. Frankland AW, Easty D: Vernal keratoconjunctivitis: An atopic disease. Trans Ophthalmol Soc UK 91:479, 1971 11. Easty D, Birkenshaw M, Merrett T et al: Immunological investigations in vernal eye disease. Trans Ophthalmol Soc UK 190:98, 1980 12. Zavaro A, Baryishak YR, Samra Z et al: Extrinsic and idiopathic vernal keratoconjunctivitis? Two cases with dissimilar
immunopathology. Br J Ophthalmol 67:742, 1983 13. Abu El-Asrar AM, Van den Oord JJ, Geboes K et al: Immunopathological study of vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol 227:374, 1989 14. Allansmith MR, Hahn GS, Simon MA: Tissue, tear, and serum IgE concentrations in vernal conjunctivitis. Am J Ophthalmol 81:506, 1976 15. Allansmith MR, Frick OL: Antibodies to grass in vernal conjunctivitis. J Allergy 34:535, 1963 16. Ballow M, Mendelson L: Specific immunoglobulin E antibodies in tear secretions of patients with
vernal conjunctivitis. J Allergy Clin Immunol 66:112, 1980 17. Ballow M, Donshik PC, Mendelson L et al: IgG specific antibodies to rye grass and ragweed pollen antigens in the
tear secretions of patients with vernal conjunctivitis. Am J Ophthalmol 95:161, 1983 18. Abelson MB, Soter NA, Simon MA: Histamine in human tears. Am J Ophthalmol 83:417, 1977 19. Udell IR, Gleich GJ, Allansmith MR et al: Eosinophil granule major basic protein and Charcot-Leyden crystal protein
in human tears. Am J Ophthalmol 92:824, 1981 20. Trocme SD, Kephart GM, Allansmith MA et al: Conjunctival deposition of eosinophil granule major basic protein in vernal
keratoconjunctivitis and contact lens-associated giant papillary
conjunctivitis. Am J Ophthalmol 108:57, 1989 21. Trocmé SD, Kephart GM, Bourne WM et al: Eosinophil granule major basic protein deposition in corneal ulcers associated
with vernal keratoconjunctivitis. Am J Ophthalmol 115:640, 1993 22. Bhan AK, Fujikawa LS, Foster CS: T-cell subsets and Langerhans cells in normal and diseased conjunctiva. Am J Ophthalmol 94:205, 1982 23. Maggi E, Biswas P, Del Prete G et al: Accumulation of Th-2-like helper T cells in the conjunctiva of patients
with vernal conjunctivitis. J Immunol 146:1169, 1991 24. Collin HB, Allansmith MR: Basophils in vernal conjunctivitis in humans: An electron microscope study. Invest Ophthalmol Vis Sci 16:858, 1977 25. Stock EL, Meisler DM: Cutaneous basophil hypersensitivity in the guinea pig conjunctiva. Curr Eye Res 2:887, 1982/1983 26. Butrus SI, Leung DYM, Gellis S et al: Vernal conjunctivitis in the hyperimmunoglobulin E syndrome. Ophthalmology 91:1213, 1984 27. Cameron JA, Al-Rajhi AA, Badr IA: Corneal ectasia in vernal keratoconjunctivitis. Ophthalmology 96:1615, 1989 28. Beigelman MN: Vernal Conjunctivitis. Los Angeles, University of Southern
California Press, 1950 29. Herbert H: Preliminary notes on the pathology and diagnosis of spring catarrh. Br
Med J II:735, 1903 30. Axenfeld T: Rapport sur le catarrhe printanier. Bull Mem Soc Fr Ophtalmol 24:1, 1907 31. Morgan G: The pathology of vernal conjunctivitis. Trans Ophthalmol Soc UK 91:467, 1971 32. Allansmith MR, Baird RS: Percentage of degranulated mast cells in vernal conjunctivitis and giant
papillary conjunctivitis associated with contact lens wear. Am J Ophthalmol 9:71, 1981 33. Easty DL, Birkenshaw M, Merrett T et al: Immunologic investigations in vernal eye disease. Trans Ophthalmol Soc UK 190:98, 1980 34. Irani AA, Schecter NM, Craig SS et al: Two types of mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA 83:4464, 1986 35. Easty D, Rice NSC, Jones B: Disodium cromoglycate (Intal) in the treatment of vernal keratoconjunctivitis. Trans Ophthalmol Soc UK 91:491, 1971 36. Bonini S, Barney NP, Schiavone M et al: Effectiveness of nedocromil sodium 2% eyedrops on clinical symptoms and
tear fluid cytology of patients with vernal conjunctivitis. Eye 65:648, 1992 37. Caldwell DR, Philippe V, Hartwich-Young R et al: Efficacy and safety of lodoxamide 0.1% vs cromolyn sodium 4% in patients
with vernal keratoconjunctivitis. Am J Ophthalmol 113:632, 1992 38. Abelson MB, Butrus S, Weston JH: Aspirin therapy in vernal conjunctivitis. Am J Ophthalmol 95:502, 1983 39. Meyer E, Kraus E, Zonis S: Efficacy of antiprostaglandin therapy in vernal conjunctivitis. Br J Ophthalmol 71:497, 1987 40. Lemrini F, Dafrallah L, Sebbahi et al: Traitement de la conjonctivite printanière
par l'aspirine. Rev Int Trach Pathol Ocul Trop Subtrop
Sante Publique 66:119, 1989 41. Gupta S, Khurana AK, Ahluwalia BK et al: Topical indomethacin for vernal keratoconjunctivitis. Acta Ophthalmol 69:95, 1991 42. Tinkelman DG, Rupp G, Kaufman H et al: Double masked, paired comparison clinical study of ketorolac tromethamine 0.5% ophthalmic
solution compared with placebo eye drops in the treatment
of seasonal allergic conjunctivitis. Surv Ophthalmol (suppl) 38:133, 1993 43. Haynes RC Jr: Adrenocorticotropic hormone; adrenocortical steroids and
their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical
hormone. In Gilman AG, Rail TW, Nies AS et al (eds): The
Pharmacological Basis of Therapeutics, 8th ed, pp 1442–1460. New
York, Pergamon Press, 1990 44. Stock EL, Pendleton RB: Pharmacological treatment of ocular allergic diseases. Int Ophthalmol Clin 33:47, 1993 45. BenEzra D, Pe'er J, Brodsky M et al: Cyclosporin eye drops for the treatment of severe vernal keratoconjunctivitis. Am J Ophthalmol 101:278, 1986 46. Bleik JH, Tabbara KF: Topical cyclosporine in vernal keratoconjunctivitis. Ophthalmology 98:1679, 1991 47. Secchi AG, Tognon MS, Leonardi A: Topical use of cyclosporine in the treatment of vernal keratoconjunctivitis. Am J Ophthalmol 110:641, 1990 48. Sankarkumar T, Panda A, Angra SK: Efficacy of cryotherapy in vernal catarrh. Am J Ophthalmol 124:253, 1992 49. Sugar HS: Tarsectomy for proliferative palpebral vernal conjunctivitis. Am J Ophthalmol 53:429, 1962 50. Cross AG: Surgical treatment of persistent vernal catarrh. Trans Ophthalmol Soc UK 79:45, 1959 51. Tse DT, Mandelbaum S, Epstein S et al: Mucous membrane grafting for severe palpebral vernal conjunctivitis. Arch Ophthalmol 101:1879, 1983 52. Buckley RJ: Vernal keratoconjunctivitis. Int Ophthalmol Clin 28:303, 1988 53. Hughes WF Jr: Beta radiation therapy in ophthalmology. Trans Am Ophthalmol Soc 50:469, 1952 |