ACUTE BACTERIAL CONJUNCTIVITIS
Acute bacterial conjunctivitis commonly begins unilaterally with irritation and tearing accompanied by the development of mucopurulent or purulent discharge and mattering of the lids. The second eye often is involved 24 to 48 hours later. Symptoms are accompanied by epibulbar and tarsal conjunctival hyperemia, and there often is punctate epithelial keratitis. There generally is minimal or no lymphadenopathy. The pathogens most commonly associated with acute bacterial conjunctivitis include Staphylococcus aureus, coagulase-negative Staphylococcus, S. pneumoniae, and H. influenzae. In children, the most common pathogens are similar, with H. influenzae and S. pneumoniae the more frequent.
S. aureus is the most common cause of bacterial conjunctivitis worldwide. This ubiquitous aerobic gram-positive organism, which colonizes humans within a few days after birth, normally is not part of the ocular flora. It is seen both singly and in pairs, and, less commonly, in clusters in conjunctival smears. It is 1 μm in diameter, and it produces cream-colored or golden colonies and complete hemolysis on blood agar. Conjunctival infection probably is caused by spread from the adjacent facial skin or nares.16 The tissue-cytologic response is polymorphonuclear. Although the clinical manifestations of infection induced by S. aureus are numerous, the organism can induce a self-limited inflammatory episode with an acute onset associated with a mucopurulent discharge. There is no seasonal incidence of infection.
S. epidermidis, a type of coagulase-negative Staphylococcus species, also may produce conjunctivitis, despite its appearance as normal flora in many individuals. Strains causing infections may elaborate toxins similar to S. aureus.17 In one study, of those with conjunctivitis and blepharitis versus controls, S. epidermidis was the most frequent species isolated from the conjunctiva and lids of both groups, whereas S. aureus was isolated only from infected patients.18
S. pneumoniae is an aerobic, encapsulated, gram-positive diplococcus carried most commonly in the upper respiratory tract of healthy children of preschool or grammar school age. It is more commonly seen in more temperate climates during the colder months of the year. The serotypes causing eye disease are those that are found in the healthy carrier. On gram-stained smears of conjunctival scrapings, the organism occurs in pairs, is lancet shaped, and elicits a substantial polymorphonuclear response. Some appear encapsulated (polysaccharide). Colonies grow on blood or chocolate agar. S. pneumoniae produces a self-limited (7 to 11 days), mucopurulent conjunctivitis that generally reaches its peak in 2 to 3 days and commonly resolves without damage to the conjunctiva. It is more common in children and may be associated with institutional epidemics. Other Streptococcal species, such as the Streptococcus viridans group also may act as pathogens. Streptococcus species usually cause a red eye and a sticky mucopurulent discharge. Subconjunctival hemorrhages and chemosis may occur. In some subjects, beta-hemolytic Streptococci may produce a severe purulent conjunctivitis that can progress to pseudomembrane or membrane formation.
H. influenzae biotype III (formerly Haemophilus aegyptius) is a fastidious, aerobic, gram-negative, coccobacillary organism that, like other bacterial causes of conjunctivitis, may be isolated from the upper respiratory tract of healthy carriers. It is found more often in children than in adults, appears with greater frequency in warmer climates, and is one of the most common cause of bilateral conjunctivitis in children. Occasionally, a few colonies of the organism may be found on the surface of the healthy eye. Because Haemophilus species require an iron protoporphyrin (X-factor) and nicotinamide adenine dinucleotide (V-factor) for growth, chocolate agar, composed of heat-disrupted erythrocytes, provides optimal growth conditions. H. influenzae produces an acute conjunctivitis that sometimes is more severe and lengthy (10 to 15 days) than conjunctivitis produced by gram-positive organisms. It reaches a peak on the third or fourth day after inoculation and is characterized by a mucopurulent discharge and fine, petechial conjunctival hemorrhages. In children aged 6 months to 3 years, the conjunctivitis may be accompanied by a bluish periorbital discoloration and swelling suggestive of preseptal cellulitis.8,19 It often is associated with fever, upper respiratory tract infection, and leukocytosis. Further, the “conjunctivitis-otitis” syndrome may result, requiring systemic treatment for the otitis media. Systemic treatment for younger children to prevent the otitis and upper respiratory tract infection has been recommended.19 The organism associated with this symptom complex is H. influenzae, type B, and may be accompanied by bacteremia and metastatic meningitis, septic arthritis, or endophthalmitis. Other strains causing acute Haemophilus conjunctivitis generally are not as virulent. In areas of endemic trachoma, Haemophilus organisms may be associated with seasonal waves of epidemic conjunctivitis.
With the exception of Moraxella lacunata, which may produce chronic follicular conjunctivitis, and S. aureus, which can progress to chronic blepharoconjunctivitis, most cases of acute bacterial conjunctivitis resolve either spontaneously or promptly with antibiotic therapy. Of note, these two organisms also may cause enlarged preauricular nodes, more characteristic of infection of other etiology.
HYPERACUTE BACTERIAL CONJUNCTIVITIS
Hyperacute conjunctivitis is so named because of its abrupt onset and copious suppurative discharge, which characteristically reaccumulates immediately after it is wiped away. The patient experiences redness and irritation with rapid progression of symptoms, including lid swelling, aching, tenderness to palpation, and purulent discharge. There is marked conjunctival hyperemia and chemosis, and an inflammatory membrane may form. Preauricular adenopathy commonly is seen. The severity of the symptoms generally prompts the patient to seek medical help early in the course of the disease. The two pathogens most commonly associated with hyperacute conjunctivitis are the Neisseria species Neisseria gonorrhoeae and Neisseria meningitidis. Other members of the Neisseria family (Neisseria catarrhalis and Neisseria sicca) frequently are isolated from the normal oropharynx, but they may be a cause of chronic conjunctivitis.
Pathogenic Neisseria organisms are coffee bean shaped, gram-negative aerobic diplococci seen within the cytoplasm of polymorphonuclear leukocytes. They are fastidious and are best grown on Thayer-Martin agar in 2% to 10% CO2 at 36°C. The pathogenicity of N. gonorrhoeae is related to its ability to attach to the epithelial surface. It is a true epithelial parasite, and by invading epithelial cells as well as polymorphonuclear leukocytes, it escapes phagocytosis. Other organisms that are capable of epithelial parasitism include Listeria monocytogenes, Corynebacterium diptheriae, and Haemophilus species.
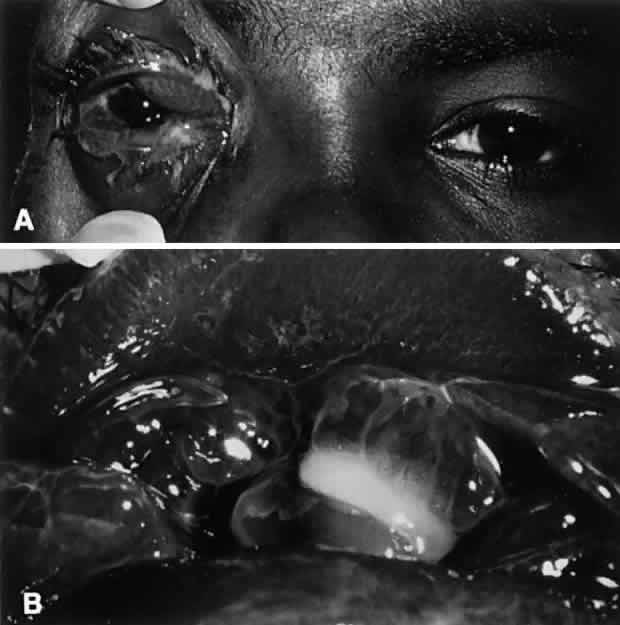
N. gonorrhoeae is by far the more common of the two Neisseria species infections and is considered to be an oculogenital disease. It is seen primarily in neonates and in sexually active adolescents and young adults. Transmission generally is from genitalia to hand to eye. A careful history of sexual activity should be taken, including contact with infected individuals and symptoms of urethritis, vaginitis, or proctitis. Initially, ocular symptoms are similar to those of acute conjunctivitis but progress quickly to include swelling of the lids, profuse tenacious yellow-green purulent discharge, hyperemia and chemosis of the conjunctiva, pain, tenderness to touch, and prominent, tender preauricular nodes (Fig. 1). In cases that go untreated, corneal involvement is a consistent feature, and for this reason, gonococcal conjunctivitis represents a clinical condition of considerable importance. Corneal involvement begins with a loss of corneal luster, or a corneal haze. This is followed by a breakdown of the corneal epithelium, most often in the periphery. An ulcerative gutter surrounded by stromal infiltrate then forms; this may progress circumferentially to form a ring abscess or may progress centrally. Such ulcers may burrow and perforate rapidly, resulting in endophthalmitis. In addition to keratoconjunctivitis, iritis, lid abscesses, dacryoadenitis, and septicemia may occur. If left untreated, conjunctival inflammation caused by these organisms may progress to involve the cornea, with peripheral ulceration, abscess formation, and ultimately, perforation.
Meningococci are carried in the oropharynx of up to 38% of healthy persons and may be the cause of epidemic meningitis. They are not commonly the cause of conjunctivitis but may cause serious conjunctival infection in children and young adults with resultant meningococcemia. The purulent conjunctivitis caused by N. meningitidis is clinically identical to that caused by gonococcal infection, but it usually is seen in younger patients and frequently is bilateral.20,21 The corneal findings generally are milder than in gonococcal infection. In addition, N. meningitidis infection may be complicated by meningococcemia, metastatic meningitis, or endogenous endophthalmitis.
CHRONIC BACTERIAL CONJUNCTIVITIS
Symptoms of chronic bacterial conjunctivitis are present for 4 weeks or more and usually consist of redness, irritation, lid excoriation, daily discharge, morning crusting, or mild mattering of the lids. Clinical signs often are nonspecific and include diffuse conjunctival hyperemia, papillae or follicles, minimal mucoid or mucopurulent discharge, and thickening of the conjunctiva. Treatment failures and recurrences are common. In some patients, specific organisms typically cause a more drawn-out course. In others, the organism simply is resistant to the antibiotic initially chosen. Masquerade syndromes are common and include viral conjunctivitis (molluscum contagiosum), lid disease (meibomian gland dysfunction, blepharoconjunctivitis, acne rosacea), allergic/atopic conjunctivitis, toxic conjunctivitis, and chronic dacryocystitis. The search for adnexal sources of infection is especially important because they may influence culture results, often may not be treated effectively by topical drops, may be caused by resistant organisms, or may require more long-term, intensive, or systemic treatment.
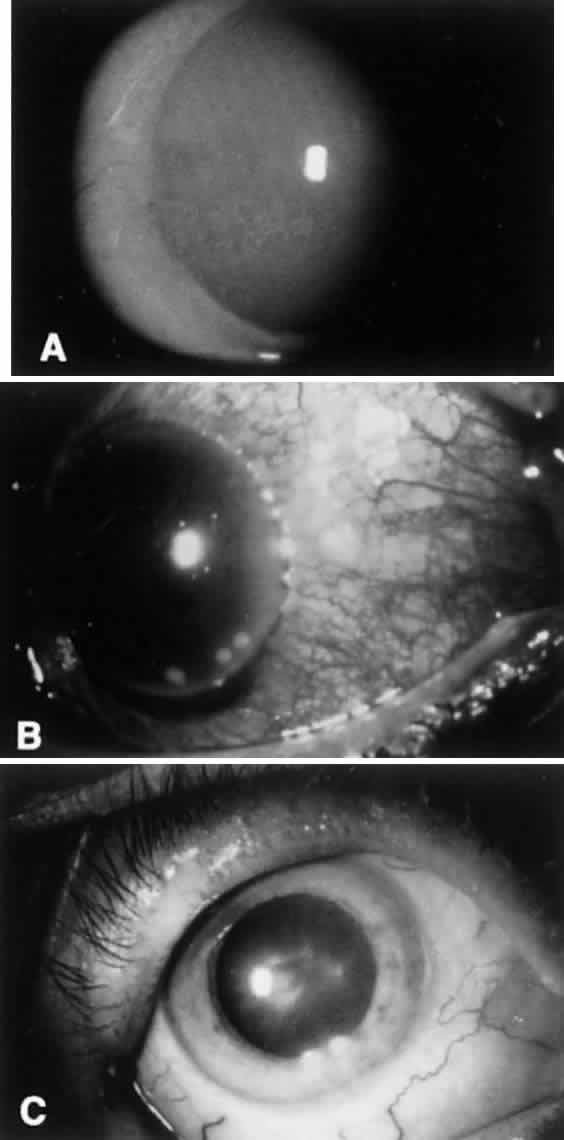
Chronic bacterial conjunctivitis is differentiated from other causes of chronic conjunctivitis on the basis of history, physical findings, and laboratory work-up. History taking should include questions regarding infection exposure, genitourinary symptoms, application of cosmetics, and instillation of topical medicines. Pressure over the lacrimal drainage system may produce a discharge indicative of dacryocystitis. The lids and canthal angles should be inspected for excoriation, ulceration, and erythema. The lids should be inspected closely for signs of anatomic abnormalities or signs of meibomianitis and blepharitis (Fig. 2). The involvement of the lids with eczematoid dermatitis, lash loss, trichiasis, collarettes, redness or telangiectasis of the lid margin, or recurrent hordeolum strongly suggest meibomian gland dysfunction. The exudate in such cases is crusted and yellowish and often encases the base of the cilia. Laboratory work-up includes culturing of the lid margin and conjunctiva to look for heavy growth of a predominant pathogen. Impression cytology with of the ocular surface is less helpful because of lack of specificity.22 Microscopic cytologic evaluation of conjunctival scrapings can add valuable information that correlates well with microbiology. Neutrophils are correlated with positive bacterial cultures, lymphocytosis with viral etiologies, characteristic basophilic inclusions with chlamydial infection, and eosinophils with allergic conjunctivitis.22
S. aureus is the organism isolated most commonly in cases of chronic bacterial conjunctivitis.23 Coagulase-negative Staphylococcus species, including S. epidermidis, may cause a similar clinical picture. Staphylococci may infect the conjunctiva primarily or may colonize the lid margin. Conjunctival inflammation occurs either by direct infection or through elaboration of exotoxins.24,25 The latter are believed to be responsible for a nonspecific conjunctivitis, punctate epithelial keratitis, phlyctenular keratoconjunctivitis, marginal corneal ulceration, and angular conjunctivitis. These entities are responsive to steroid-antibiotic combinations, which serve to decrease inflammation as well as bacterial counts. Such preparations, however, must be employed with caution.
Staphylococcus species conjunctivitis may result in inferior cornea fine punctate epithelial keratitis, which may be the result of bacterial toxigenicity. This may be responsible for a foreign-body sensation and is most prominent during the morning hours. Phlyctenular keratitis may complicate chronic Staphylococcus blepharoconjunctivitis. The gelatinous nodular lesions occur most commonly at the limbus, but they may be wholly corneal or conjunctival in location and acutely painful.
Marginal corneal ulceration due to hypersensitivity to staphylococcal exotoxin also may occur. Findings include the formation of single or multiple gray-white infiltrates with or without subsequent overlying epithelial breakdown. These are most often seen along the inferior limbus between the 4- and 8-o'clock positions. The ulcers frequently are associated with paralimbal conjunctival vascular engorgement and generally are culture negative (Fig. 3).
Maceration, excoriation, and crusting of the lateral canthal angle, along with localized, lateral epibulbar injection indicate the presence of angular blepharitis, a form of chronic conjunctivitis most commonly caused by Staphylococcus or Moraxella. Bacterial elaboration of dermonecrotoxins produce the characteristic findings.
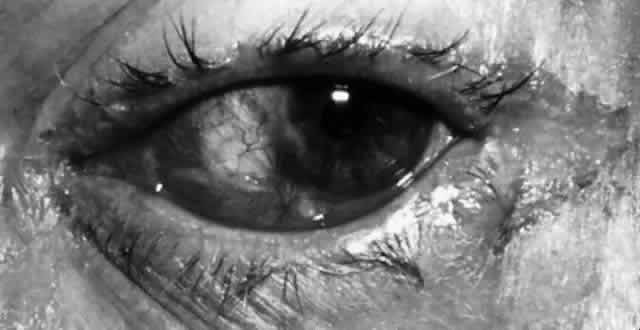
M. lacunata organisms are large, symmetric, gram-negative diplobacilli that are cultured readily on blood or chocolate agar. The Moraxella organisms may retain gentian violet in the Gram staining process and thus may appear gram positive. In the conjunctiva, Moraxella organisms do not evoke a significant polymorphonuclear response. Infection may result in a chronic follicular conjunctivitis, angular conjunctivitis, or an enlarged preauricular lymph node (Fig. 4). Other organisms, especially in the elderly or immunocompromised, also may be implicated. These organisms are largely enteric bacteria and include Proteus mirabilis, Escherichia coli, Branhamella catarrhalis, Klebsiella pneumoniae, Serratia marcescens, and Pseudomonas. Serratia marcescens organisms are gram-negative rods with parallel sides and rounded ends. N. catarrhalis, which normally is found in the upper respiratory tract, also may be a cause of chronic conjunctivitis. Pseudomonas organisms appear as single rods that are straight or slightly curved. Although all of these organisms occasionally can be isolated from the ocular surface, they are located in much larger numbers in the presence of an infection.
|