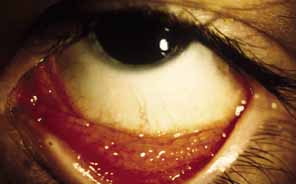
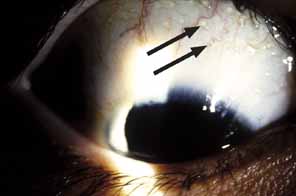
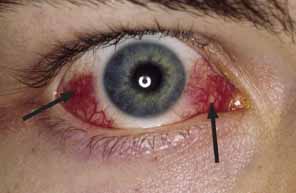
1. Parinaud H: Conjonctivité infectieuse transmisé par les animaux. Ann Ocul 101:252, 1889 2. Giladi M, Avidor B, Kletter Y, Abulafia S, Slater LN, Welch DF, et al: Cat scratch disease: The rare role of Afipia felis. Journal of Clinical Microbiology 36:2499–502, 1998 3. Bergmans AM, Groothedde JW, Schellekens JF, van Embden JD, Ossewaarde JM, Schouls LM: Etiology of cat scratch disease: Comparison of polymerase chain reaction
detection of Bartonella (formerly Rochalimaea) and Afipia felis DNA with serology and skin tests. Journal of Infectious Diseases 171:916–23, 1995 4. Carithers HA: Cat-scratch disease. An overview based on a study of 1,200 patients. American Journal of Diseases
of Children 139:1124–33, 1985 5. Margileth AM: Cat scratch disease: No longer a diagnostic dilemma. Seminars in Veterinary Medicine & Surgery (Small Animal) 6:199–202, 1991 6. Ormerod LD, Dailey JP: Ocular manifestations of cat-scratch disease. Current Opinion in Ophthalmology 10:209–16, 1999 7. Kordick DL, Wilson KH, Sexton DJ, Hadfield TL, Berkhoff HA, Breitschwerdt EB: Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. Journal of Clinical Microbiology 33:3245–51, 1995 8. Murano I, Yoshii H, Kurashige H, Sugio Y, Uchida M, Shinohara T, et al: Three children with systemic cat scratch disease. Kansenshogaku Zasshi—Journal of the Japanese Association for Infectious
Diseases 73:248–52, 1999 9. Cunningham ET, Koehler JE: Ocular bartonellosis. Am J Ophthalmol 130:340–9, 2000 10. Murakami K, Tsukahara M, Tsuneoka H, Iino H, Ishida C, Tsujino K, et al: Cat scratch disease: Analysis of 130 seropositive cases. Journal of Infection & Chemotherapy 8:349–52, 2002 11. Gabler B, Linde HJ, Reischl U, Lohmann CP: [Disciform keratatis caused by Bartonella henselae infection: detection of a rare ocular complication of cat-scratch
disease with PCR]. Klinische Monatsblatter fur Augenheilkunde 217:299–302, 2000 12. Resto-Ruiz S, Burgess A, Anderson BE: The role of the host immune response in pathogenesis of Bartonella henselae. DNA & Cell Biology 22:431–40, 2003 13. Torok L, Viragh SZ, Borka I, Tapai M: Bacillary angiomatosis in a patient with lymphocytic leukaemia. British Journal of Dermatology 130:665–8, 1994 14. Koehler JE, Sanchez MA, Tye S, Garrido-Rowland CS, Chen FM, Maurer T, et al: Prevalence of Bartonella infection among human immunodeficiency virus-infected patients
with fever. Clinical Infectious Diseases 37:559–66, 2003 15. Frean J, Arndt S, Spencer D: High rate of Bartonella henselae infection in HIV-positive outpatients in Johannesburg, South Africa. Transactions of the Royal Society of Tropical Medicine & Hygiene 96:549–50, 2002 16. Schwartzman WA, Patnaik M, Angulo FJ, Visscher BR, Miller EN, Peter JB: Bartonella (Rochalimaea) antibodies, dementia, and cat ownership among men infected with
human immunodeficiency virus. Clinical Infectious Diseases 21:954–9, 1995 17. Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS: The agent of bacillary angiomatosis. An approach to the identification
of uncultured pathogens [comment]. New England Journal of Medicine 323:1573–80, 1990 18. Starck T, Madsen BW: Positive polymerase chain reaction and histology with borderline serology
in Parinaud's oculoglandular syndrome. Cornea 21:625–7, 2002 19. Margolis B, Kuzu I, Herrmann M, Raible MD, Hsi E, Alkan S: Rapid polymerase chain reaction-based confirmation of cat scratch
disease and Bartonella henselae infection. Archives of Pathology & Laboratory Medicine 127:706–10, 2003 20. Labalette P, Bermond D, Dedes V, Savage C: Cat-scratch disease neuroretinitis diagnosed by a polymerase chain
reaction approach. American Journal of Ophthalmology 132:575–6, 2001 21. Hara H, Ito K, Akimoto M, Suzuki H, Asai S, Maruyama S: Detection of Bartonella henselae DNA using polymerase chain reaction assay in patient with cat scratch
disease. Acta Dermato-Venereologica 83:67–8, 2003 22. Dalton MJ, Robinson LE, Cooper J, Regnery RL, Olson JG, Childs JE: Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national
referral center. Archives of Internal Medicine 155:1670–6, 1995 23. Bergmans AM, Peeters MF, Schellekens JF, Vos MC, Sabbe LJ, Ossewaarde JM, et al: Pitfalls and fallacies of cat scratch disease serology: Evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay [comment]. Journal of Clinical Microbiology 35:1931–7, 1997 24. Giladi M, Kletter Y, Avidor B, Metzkor-Cotter E, Varon M, Golan Y, et al: Enzyme immunoassay for the diagnosis of cat-scratch disease defined
by polymerase chain reaction. Clinical Infectious Diseases 33:1852–8, 2001 25. Sander A, Berner R, Ruess M: Serodiagnosis of cat scratch disease: Response to Bartonella henselae in children and a review of diagnostic methods. European Journal of Clinical Microbiology & Infectious Diseases 20:392–401, 2001 26. Fanous MM, Margo CE: Parinaud's oculoglandular syndrome simulating lymphoma. American Journal of Ophthalmology 112:344–5, 1991 27. Conrad DA: Treatment of cat-scratch disease. Current Opinion in Pediatrics 13:56–9, 2001 28. Lerdluedeeporn P, Krogstad P, Roberts RL, Stiehm ER: Oral corticosteroids in cat-scratch disease. Clinical Pediatrics 42:71–3, 2003 29. Bass JW, Freitas BC, Freitas AD, Sisler CL, Chan DS, Vincent JM, et al: Prospective randomized double blind placebo-controlled evaluation
of azithromycin for treatment of cat-scratch disease [comment]. Pediatric Infectious Disease Journal 17:447–52, 1998 30. Mui BS, Mulligan ME, George WL: Response of HIV-associated disseminated cat scratch disease to treatment
with doxycycline. American Journal of Medicine 89:229–31, 1990 31. Thompson S, Omphroy L, Oetting T: Parinaud's oculoglandular syndrome attributable to an encounter with
a wild rabbit. American Journal of Ophthalmology 131:283–4, 2001 32. Chappell CW, Brainard J, Shock JP: Oculoglandular tularemia—a case report. Journal of the Arkansas Medical Society 78:128–30, 1981 33. Halperin SA, Gast T, Ferrieri P: Oculoglandular syndrome caused by Francisella tularensis. Clinical Pediatrics 24:520–2, 1985 34. Peter R, Banyai T: Erythema nodosum revealing oculoglandular tularemia. Dermatology 202:79–80, 2001 35. Steinemann TL, Sheikholeslami MR, Brown HH, Bradsher RW: Oculoglandular tularemia. Archives of Ophthalmology 117:132–3, 1999 36. Parssinen O, Rummukainen M: Acute glaucoma and acute corneal oedema in association with tularemia. Acta Ophthalmologica Scandinavica 75:732–4, 1997 37. Hampton DE, Adesina A, Chodosh J: Conjunctival sporotrichosis in the absence of antecedent trauma. Cornea 21:831–3, 2002 38. Trent JT, Kirsner RS: Identifying and treating mycotic skin infections. Advances in Skin & Wound Care 16:122–9; quiz 130–1, 2003 39. Sobel JD: Practice guidelines for the treatment of fungal infections. For the Mycoses
Study Group. Infectious Diseases Society of America. Clinical Infectious Diseases 30:652, 2000 40. Cook CD, Hainsworth M: Tuberculosis of the conjunctiva occurring in association with a neighbouring
lupus vulgaris lesion. British Journal of Ophthalmology 74:315–6, 1990 41. Whitford J, Hansman D: Primary tuberculosis of the conjunctiva. Medical Journal of Australia 1:486–7, 1977 42. Horsburgh CR Jr, Feldman S, Ridzon R: Infectious Diseases Society of A: Practice guidelines for the treatment
of tuberculosis. Clinical Infectious Diseases 31:633–9, 2003 43. Spektor FE, Eagle RC Jr , Nichols CW: Granulomatous conjunctivitis secondary to Treponema pallidum. Ophthalmology 88:863–5, 1981 44. Maguire LJ, Campbell RJ, Edson RS: Coccidioidomycosis with necrotizing granulomatous conjunctivitis. Cornea 13:539–42, 1994 45. Wood TR: Ocular coccidioidomycosis. Report of a case presenting as Parinaud's
oculoglandular syndrome. American Journal of Ophthalmology 64:Suppl:587–90, 1967 46. Slack JW, Hyndiuk RA, Harris GJ, Simons KB: Blastomycosis of the eyelid and conjunctiva. Ophthalmic Plastic & Reconstructive Surgery 8:143–9, 1992 47. Galgiani JN: Coccidioidomycosis: A regional disease of national importance. Rethinking
approaches for control. Annals of Internal Medicine 130:293–300, 1999 48. Rodenbiker HT, Ganley JP: Ocular coccidioidomycosis. Survey of Ophthalmology 24:263–90, 1980 49. Meisler DM, Bosworth DE, Krachmer JH: Ocular infectious mononucleosis manifested as Parinaud's oculoglandular
syndrome. American Journal of Ophthalmology 92:722–6, 1981 50. Wilhelmus KR: Ocular involvement in infectious mononucleosis. American Journal of Ophthalmology 91:117–8, 1981 51. Ho AC, Rapuano CJ: Pasteurella multocida keratitis and corneal laceration from a cat scratch. Ophthalmic Surgery 24:346–8, 1993 52. Chin GN, Noble RC: Ocular involvement in Yersinia enterocolitica infection presenting as Parinaud's
oculoglandular syndrome. American Journal of Ophthalmology 83:19–23, 1977 53. Srinivasan A, Kraus CN, DeShazer D, Becker PM, Dick JD, Spacek L, et al: Glanders in a military research microbiologist [comment]. New England Journal of Medicine 345:256–8, 2001 54. Buus DR, Pflugfelder SC, Schachter J, Miller D, Forster RK: Lymphogranuloma venereum conjunctivitis with a marginal corneal perforation. Ophthalmology 95:799–802, 1988 55. Macnie J: Ocular lymphogranuloma venereum. Arch Ophthalmol 25:255, 1941 56. Martin X, Uffer S, Gailloud C: Ophthalmia nodosa and the oculoglandular syndrome of Parinaud. British Journal of Ophthalmology 70:536–42, 1986 57. Bernardino CR, Rapuano C: Ophthalmia nodosa caused by casual handling of a tarantula. CLAO Journal 26:111–2, 2000 58. Shrum KR, Robertson DM, Baratz KH, Casperson TJ, Rostvold JA: Keratitis and retinitis secondary to tarantula hair. Archives of Ophthalmology 117:1096–7, 1999 59. Watts P, McPherson R, Hawksworth NR: Tarantula keratouveitis. Cornea 19:393–4, 2000 60. Weinberg JC, Eagle RC Jr , Font RL, Streeten BW, Hidayat A, Morris DA: Conjunctival synthetic fiber granuloma. A lesion that resembles conjunctivitis
nodosa. Ophthalmology 91:867–72, 1984 61. Lueder GT, Matsumoto B, Smith ME: Pathological case of the month. Synthetic fiber granuloma (“Teddy
Bear Granuloma”). Archives of Pediatrics & Adolescent Medicine 150:327–8, 1996 |