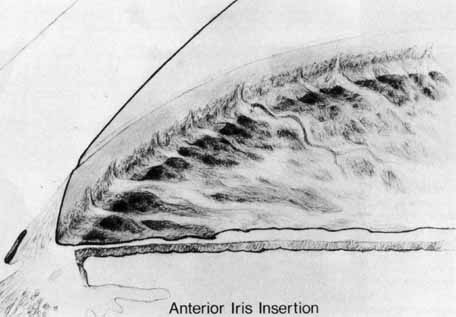
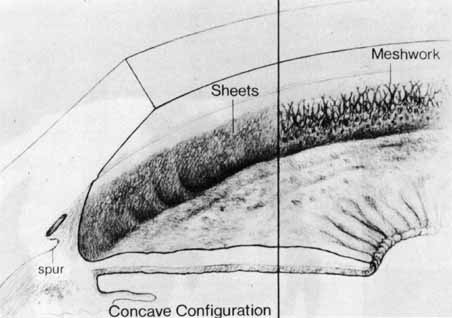
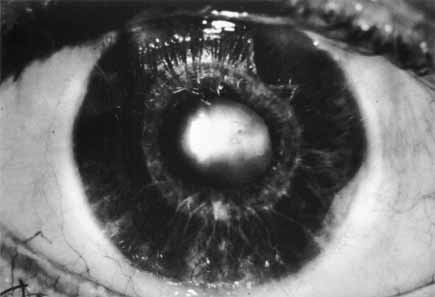
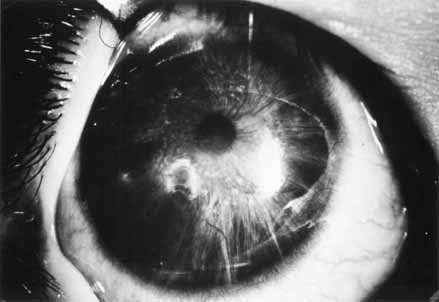
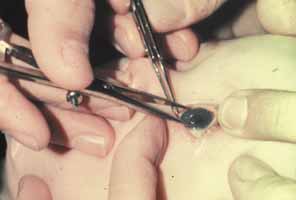
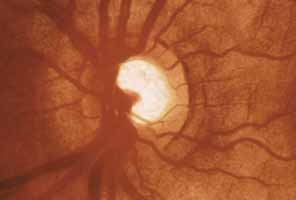
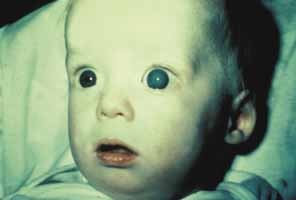
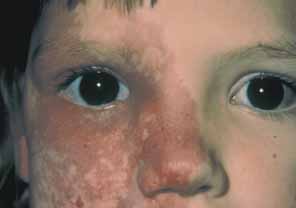
1. Shaffer RN, Weiss DI: Congenital and Pediatric Glaucomas. St. Louis: CV Mosby, 1970 2. Hoskins HD Jr, Hetherington J Jr, Shaffer RN, et al: Developmental glaucoma: diagnosis and classification. Proceedings of the New Orleans Academy of Ophthalmology Glaucoma Symposium. St. Louis: CV Mosby, 1981 3. Hoskins HD Jr, Shaffer RN, Hetherington J Jr: Anatomical classification of the developmental glaucomas. Arch Ophthalmol 102:1331, 1984 4. Luntz MH: Congenital, infantile, juvenile glaucoma. Ophthalmology 86:793, 1979 5. Law SK, Bui D, Caprioli J: Serial axial length measurements in congenital glaucoma. Am J Ophthalmol 132:926, 2001 6. Shaffer RN, Hetherington J: The glaucomatous disc in infants: a suggested hypothesis for disc cupping. Trans Am Acad Ophthalmol 73:923, 1969 7. Quigley HA: Childhood glaucoma: results with trabeculotomy and study of reversible
cupping. Ophthalmology 89:219, 1982 8. Bakunowicz-Lazarczyk A, Sulkowska M, Sulkowski S, et al: Ultrastructural changes in the trabecular meshwork of congenital glaucoma. J Submicrosc Cytol Pathol 33:17, 2001 9. Tawara A, Inomata H: Developmental immaturity of the trabecular meshwork in congenital glaucoma. Am J Ophthalmol 92:508, 1981 10. deLuise VP, Anderson DR: Primary infantile glaucoma (congenital glaucoma). Surv Ophthalmol 28:1, 1983 11. Borcon AF, Katsumi O, Hirose T: Tonometry in pediatric patients: a comparative study among Tono-pen, Perkins, and
Schiötz tonometers. J Pediatr Ophthalmol Strabismus 32:373, 1995 12. Eisenberg DL, Sherman BG, McKowan CA, et al: Tonometry in adults and children. A monometric evaluation of pneumatonometry, applanation, and
TonoPen in vitro and in vivo. Ophthalmology 105:1173, 1998 13. Anderson J: Hydrophthalmia or Congenital Glaucoma: Its Causes, Treatment and Outlook. London: Cambridge University Press, 1939 14. Richardson KT, Shaffer RN: Optic nerve cupping in congenital glaucoma. Am J Ophthalmol 62:507, 1966 15. de Souza EC, Berezovsky A, Morales PHA, et al: Visual field defects in children with congenital glaucoma. J Pediatr Ophthalmol Strabismus 37:266, 2000 16. Shaffer RN: Genetics and the congenital glaucomas. Am J Ophthalmol 60:981, 1965 17. Jay MR, Phil M, Rice NSC: Genetic implications of congenital glaucoma. Metab Ophthalmol 2:257, 1978 18. Merin S, Morin D: Heredity of congenital glaucoma. Br J Ophthalmol 56:414, 1972 19. Michels-Rautenstrauss KG, Mardin CY, Zenker M, et al: Primary congenital glaucoma: three case reports on novel mutations in the
GLC3A (CYP1B1) gene. J Glaucoma 10:354, 2001 20. Sarfarazi M, Akarsu AN, Hossain A, et al: Assignment of a locus (GLC3A) for primary congenital glaucoma (buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics 30:171, 1995 21. Akarsu AN, Turacli ME, Aktan SG, et al: A second locus (GLC3B) for primary congenital glaucoma (buphthalmos) maps
to the 1p36 region. Hum Mol Genet 5:1199, 1996 22. Polansky JR, Nguyen TD: The TIGR gene, pathogenic mechanisms, and other recent advances in glaucoma
genetics. Curr Opin Ophthalmol 9:15, 1998 23. Bejjani BA, Lewis RA, Tomey KF, et al: Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant
cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet 62:325, 1998 24. Mashima Y, Suzuki Y, Sergeev Y, et al: Novel cytochrome P4501B1 (CYP1B1) gene mutations in Japanese
patients with primary congenital glaucoma. Invest Ophthalmol Vis Sci 42:2211, 2001 25. Shaffer RN: Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis). Trans Am Ophthalmol Soc 80:321, 1982 26. Hoskins HD Jr, Shaffer RN, Hetherington J: Goniotomy vs trabeculotomy. J Pediatr Ophthalmol Strabismus 21:153, 1984 27. Broughton WL, Parks MM: An analysis of treatment of congenital glaucoma by goniotomy. Am J Ophthalmol 91:566, 1981 28. Mattox C, Walton DS: Hereditary primary childhood glaucomas. Int Ophthalmol Clin 33:121, 1993 29. Fraumeni JH: The aniridia-Wilms' tumor syndrome. Birth Defects 5:198, 1969 30. Riccardi VM, Sujansky E, Smith AC, et al: Chromosomal imbalance in the aniridia-Wilms' tumor association: 11p
interstitial deletion. Pediatrics 100:574, 1982 31. Riccardi VM, Hittner HM, Francke U, et al: The aniridia-Wilms' tumor association: the critical role of
chromosome band 11p13. Cancer Genet Cytogenet 2:131, 1980 32. Layman PR, Anderson RA, Flynn JT: Frequent occurrence of hypoplastic optic disks in patients with aniridia. Am J Ophthalmol 77:513, 1974 33. Nelson LB, Spaeth GL, Nowinski TS, et al: Aniridia: a review. Surv Ophthalmol 28:621, 1984 34. Grant M, Walton D: Progressive change in the angle in cogenital aniridia with development
of glaucoma. Am J Ophthalmol 78:842, 1974 35. Walton DS: Aniridic glaucoma: the result of goniosurgery to prevent and treat this
problem. Trans Am Ophthalmol Soc 84:59, 1986 36. Susac JO, Smith JL, Scelfo RJ: The “tomato-catsup” fundus in Sturge-Weber syndrome. Arch Ophthalmol 92:69, 1974 37. Phelps CD: The pathogenesis of glaucoma in Sturge-Weber syndrome. Ophthalmology 85:276, 1978 38. Lindenauer SM: The Klippel-Trenaunay syndrome. Ann Surg 162:303, 1965 39. Barker D, Wright E, Nguyen K, et al: Gene for von Recklinghausen neurofibromatosis is in the pericentromeric
region of chromosome 17. Science 236:1100, 1987 40. Grant WM, Walton DS: Distinctive gonioscopic findings in glaucoma due to neurofibromatosis. Arch Ophthalmol 79:127, 1968 41. Cross HE, Jensen AD: Ocular manifestations in Marfan's syndrome and homocystinuria. Am J Ophthalmol 75:405, 1973 42. Burian HM, Alien L: Histologic study of the chamber angle in patients with Marfan's syndrome. Arch Ophthalmol 65:323, 1961 43. Smith JL, Stowe FR: The Pierre Robin syndrome (glossoptosis, micrognathia, cleft palate): a
review of 39 cases with emphasis on ocular lesions. J Pediatr 27:128, 1961 44. Alward WLM: Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol 130:110, 2000 45. Craig JE, Mackey DA: Glaucoma genetics: where are we? Where will we go? Curr Opin Ophthalmol 10:26, 1999 46. Shields MB, Buckley E, Klintworth GK, et al: Axenfeld-Rieger syndrome. A spectrum of developmental disorders. Surv Ophthalmol 29:387, 1985 47. Waring GO III, Rodrigues MM, Laibson PR: Anterior chamber cleavage syndrome: a stepladder classification. Surv Ophthalmol 29:3, 1975 48. Wadelius C, Fagerholm P, Petterson U, et al: Lowe oculocerebrorenal syndrome: DNA-based linkage of the gene to
Xq24–26, using tightly linked flanking markers and the correlation
to lens examination in carrier diagnosis. Am J Hum Genet 44:241, 1989 49. Lowe CU, Terrey M, MacLachlan EA: Organic aciduria, decreased renal ammonia production, hydrophthalmos, and
mental retardation: a clinical entity. Am J Dis Child 83:164, 1952 50. Johnson VP, Grayson M, Christian JC: Dominant microspherophakia. Arch Ophthalmol 85:534, 1971 51. Sarfarazi M, Stoilov I: Molecular genetics of primary congenital glaucoma. Eye 14:422, 2000 52. Rubinstein JH, Taybi H: Broad thumbs and toes and facial abnormalities. Am J Dis Child 105:588, 1963 53. Shihab ZM: Pediatric glaucoma in Rubinstein-Taybi syndrome. Glaucoma 3:288, 1984 54. Reese AB: Persistent hyperplastic primary vitreous. Am J Ophthalmol 40:317, 1955 55. Smith RE, Maumenee AE: Persistent hyperplastic primary vitreous: results of surgery. Trans Am Acad Ophthalmol Otol 78:911, 1974 56. Johnson CP, Keech RV: Prevalence of glaucoma after surgery for PHPV and infantile cataracts. J Pediatr Ophthalmol Strabismus 33:14, 1996 57. Weatherhill JR, Hart CT: Familial hypoplasia of the iris stroma associated with glaucoma. Br J Ophthalmol 53:433, 1969 58. Martin JP, Zorab EC: Familial glaucoma. Br J Ophthalmol 58:536, 1974 59. Taylor RH, Ainsworth JR, Evans AR, et al: The epidemiology of pediatric glaucoma: the Toronto experience. J AAPOS 3:308, 1999 60. Asrani S, Freedman S, Hasselblad V, et al: Does primary intraocular lens implantation prevent 'aphakic' glaucoma
in children? J AAPOS 3:33, 2000 61. Hartnett ME, Gilbert MM, Richardson TM, et al: Anterior segment evaluation of infants with retinopathy of prematurity. Ophthalmology 97:122, 1990 62. Beck AD: Diagnosis and management of pediatric glaucoma. Ophthalmol Clin North Am 14:501, 2001 63. Hoskins HD Jr, Hetherington J Jr, Magee SD, et al: Clinical experience with timolol in childhood glaucoma. Arch Ophthalmol 103:1163, 1985 64. Boger WP III, Walton DS: Timolol in uncontrolled childhood glaucomas. Ophthalmology 88:253, 1981 65. Portellos M, Buckley EG, Freedman SF: Topical versus oral carbonic anhydrase inhibitor therapy for pediatric
glaucoma. J AAPOS 2:43, 1998 66. Carlsen JO, Zabriskie NA, Kwon YH, et al: Apparent central nervous system depression in infants after the use of
topical brimonidine. Am J Ophthalmol 128:255, 1999 67. Sztajnbok J: Failure of naloxone to reverse brimonidine-induced coma in an infant. J Pediatr 140:485, 2002 68. Brown SM: Increased iris pigment in a child due to latanoprost. Arch Ophthalmol 116:1683, 1998 69. Russell-Eggitt IM, Rice NSC, Jay B, et al: Relapse following goniotomy for congenital glaucoma due to trabecular dysgenesis. Eye 6:197, 1992 70. Bayraktar S, Koseoglu T: Endoscopic goniotomy with anterior chamber maintainer: surgical technique
and one-year results. Ophthalmic Surg Lasers 32:496, 2001 71. Joos KM, Alward WLM, Folberg R: Experimental endoscopic goniotomy: a potential treatment for primary infantile
glaucoma. Ophthalmology 100:1066, 1993 72. Meyer G, Scwenn O, Pfeiffer N, et al: Trabeculotomy in congenital glaucoma. Graefe's Arch Clin Exp Ophthalmol 238:207, 2000 73. Khaw PT: What is the best primary surgical treatment for the infantile glaucomas? Br J Ophthalmol 80:495, 1996 74. Mendicino ME, Lynch MG, Drack A, et al: Long-term surgical and visual outcomes in primary congenital glaucoma: 360 trabeculotomy
versus goniotomy. J AAPOS 4:205, 2000 75. Anderson DR: Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology 90:805, 1983 76. McPherson SD Jr, Berry DP: Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol 95:427, 1983 77. McPherson SD, McFarland D: External trabeculotomy for developmental glaucoma. Ophthalmology 87:302, 1980 78. Williams RD, Hoskins HD, Shaffer RN: Trabeculodialysis for inflammatory glaucoma: a review of 25 cases. Ophthalmic Surg 23:36, 1992 79. Beauchamp GR, Parks MM: Filtering surgery in children: barriers to success. Ophthalmology 86:170, 1979 80. Gressel MG, Heuer DK, Parrish RK: Trabeculectomy in young patients. Ophthalmology 91:1242, 1984 81. Zalish M, Leiba H, Oliver M: Subconjunctival injection of 5-fluorouracil following trabeculectomy
for congenital and infantile glaucoma. Ophthalmic Surg 23:203, 1992 82. Mockevac M, Erzurum S, Goldenfeld M, et al: Trabeculectomy with intraoperative mitomycin-C in pediatric patients. American
Academy of Ophthalmology Abstract. Ophthalmology 100(suppl):134, 1993 83. Susanna R Jr, Oltrogge EW, Carani JCE, et al: Mitomycin as adjunct chemotherapy with trabeculectomy in congenital and
developmental glaucoma. J Glaucoma 4:151, 1995 84. Mandal AK, Walton DS, John T, et al: Mitomycin C-augmented trabeculectomy in refractory congenital glaucoma. Ophthalmology 104:996, 1997 85. Al-Hazmi A, Zwaan J, Awad A, et al: Effectiveness and complications of Mitomycin C use during pediatric glaucoma
surgery. Ophthalmology 105:1915, 1998 86. Sidoti PA, Belmonte SJ, Liebmann JM, et al: Trabeculectomy with Mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology 107:422, 2000 87. Zalish M, Leiba H, Oliver M: Subconjunctival injection of 5-Fluorouracil following trabeculectomy
for congenital and infantile glaucoma. Ophthalmic Surg 23:203, 1992 88. Azuara-Blanco A, Wilson RP, Spaeth GL, et al: Filtration procedures supplemented with mitomycin C in the management of
childhood glaucoma. Br J Ophthalmol 83:151, 1999 89. Mullaney PB, Selleck C, Al-Awad A, et al: Combined trabeculotomy and trabeculectomy as an initial procedure in uncomplicated
congenital glaucoma. Arch Ophthalmol 117:457, 1999 90. Elder MJ: Combined trabeculotomy-trabeculectomy compared with primary trabeculectomy
for congenital glaucoma. Br J Ophthalmol 78:745, 1994 91. Mandal AK, Naduvilath TJ, Jayagandan A: Surgical results of combined trabeculotomy-trabeculectomy for developmental
glaucoma. Ophthalmology 105:974, 1998 92. Munoz M, Tomey KF, Traverso C, et al: Clinical experience with the Molteno implant in advanced infantile glaucoma. J Pediatr Ophthalmol Strabismus 28:68, 1991 93. Eid TE, Katz LJ, Spaeth GL, et al: Long-term effects of tube-shunt procedures on management
of refractory childhood glaucoma. Ophthalmology 104:1011, 1997 94. Netland PA, Walton DS: Glaucoma drainage implants in pediatric patients. Ophthalmic Surg 24:723, 1993 95. Hill RA, Heuer DK, Baerveldt G, et al: Molteno implantation for glaucoma in young patients. Ophthalmology 98:1042, 1991 96. Billson F, Thomas R, Aylward W: The use of two-stage Molteno implants in developmental glaucoma. J Pediatr Ophthalmol Strabismus 26:3, 1989 97. Nesher R, Sherwood MB, Kass MA, et al: Molteno implants in children. J Glaucoma 1:228, 1992 98. Donahue SP, Keech RV, Munden P, et al: Baerveldt implant surgery in the treatment of advanced childhood glaucoma. J AAPOS 1:41, 1997 99. Djodeyre MR, Calvo JP, Gomez JA: Clinical evaluation and risk factors of time to failure of Ahmed glaucoma
valve implant in pediatric patients. Ophthalmology 108:614, 2001 100. Englert JA, Freedman SF, Cox TA: The Ahmed valve in refractory pediatric glaucoma. Am J Ophthalmol 127:34, 1999 101. Coleman AL, Hill R, Wilson MR, et al: Initial clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol 120:23, 1995 102. Luke C, Dietlein TS, Jacobi PC, et al: Risk profile of deep sclerectomy for treatment of refractory congenital
glaucomas. Ophthalmology 109:1066, 2002 103. Kozobolis VP, Christodoulakis EV, Tzanakis N, et al: Primary deep sclerectomy versus primary deep sclerectomy with the use of
mitomycin C in primary open angle glaucoma. J Glaucoma 11:287, 2002 104. Wagle NS, Freedman SF, Buckley EG, et al: Long-term outcome of cyclocryotherapy for refractory pediatric glaucoma. Ophthalmology 105:1921, 1998 105. Hampton C, Shields MB: Transscleral neodymium:YAG cyclophotocoagulation: a histological study
of human autopsy eyes. Arch Ophthalmol 106:1121, 1988 106. Trope GE, Steven MA: Mid-term effects of Nd:YAG transscleral cyclocoagulation in glaucoma. Ophthalmology 97:73, 1990 107. Phelan MJ, Higginbotham EJ: Contact transscleral Nd:YAG laser cyclophotocoagulation for the treatment
of refractory pediatric glaucoma. Ophthalmic Surg Lasers 26:401, 1995 108. Schuman JS, Puliafito CA, Allingham RR, et al: Contact transscleral continuous wave neodymium:YAG laser cyclophotocoagulation. Ophthalmology 97:571, 1990 109. Kirwan JF, Shah P, Khaw PT: Diode laser photocoagulation: role in the management of refractory pediatric
glaucomas. Ophthalmology 109:316, 2002 110. Sabates R: Choroiditis compatible with the histopathologic diagnosis of sympathetic
ophthalmia following cyclocryotherapy of neovascular glaucoma. Ophthalmic Surg 19:176, 1988 111. Brown SVL, Higginbotham E, Tessler SH: Sympathetic ophthalmia following Nd:YAG cyclotherapy. Ophthalmic Surg 21:736, 1990 |