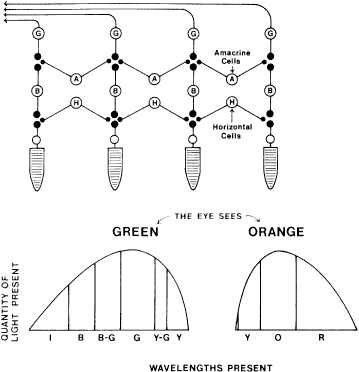
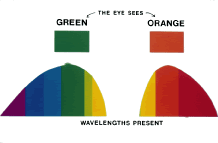
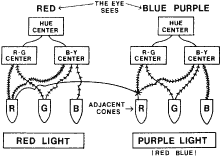
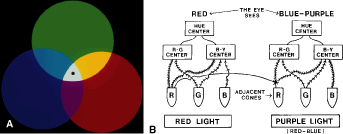
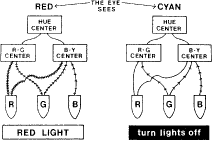
TEST CONDITIONS As previously mentioned, the apparent color of an object depends on the
ambient light. For example, a red cliff will appear redder at sunset
because the longer (red) wavelengths penetrate the atmosphere better. For
this reason, all color vision tests must be given under standard conditions. The
Illuminant “C” lamp, produced by the Macbeth
Daylighting Corporation, provides approximately the same quantity of
all wavelengths. If this is unavailable, daylight or fluorescent lighting
is next best. Incandescent lamps are unacceptable because they provide
far more energy in the long wavelengths than they do in the short
wavelengths. PSEUDOISOCHROMATIC PLATES Pseudoisochromatic plates use small colored dots that are arranged among
other dots that are either their complement or gray, so that a trichromat
can perceive a number, letter, or geometric pattern. The colors
selected are along the confusion lines of protans and deutans. For example, a
green numeral 7 and a blue-green numeral 4 may be set among red
and red purple or gray dots. Because a protan sees both blue green and
red as gray, he or she cannot see the 4 but can see the 7, which appears
to be light yellow. A deutan cannot see the 7 but can see the 4. It
appears slightly bluer than the other dots, which appear gray. Some
sets of pseudoisochromatic plates use dots of varying saturation to
estimate the severity of the color vision defect. Many sets are designed
merely to help diagnose congenital red-green deficiency and cannot
help distinguish between protans and deutans. Other sets can aid in distinguishing
between protans and deutans. Still other sets can diagnose
the severity of these abnormalities. Only a few sets are designed to
diagnose tritan defects. It must be emphasized that most pseudoisochromatic plates were designed
for the diagnosis of congenital defects. A person with acquired defects
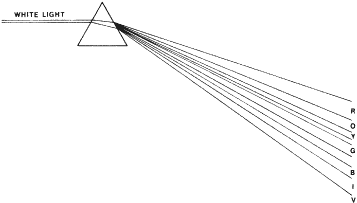
may or may not correctly identify the figures. NAGEL ANOMALOSCOPE The Nagel anomaloscope uses prisms to separate white light into the spectral
colors. Narrow slits allow narrow-band colors to be seen by the
subject, who is asked to match a 589-nm yellow with a mixture of a 545-nm
green and a 640-nm red. Looking into the instrument, the subject sees
a split field. He or she can control the intensity (luminance) of
the yellow, which is in the bottom half. The red and the green completely
overlap each other in the top half. Their luminance is fixed. The
subject can vary the relative quantity of the red and green. Protanomalous
persons use too much red to make the match, and deuteranomalous persons
use too much green. FARNSWORTH-MUNSELL TESTS Farnsworth-Munsell tests use Munsell color chips mounted in caps. The colors
differ only in hue. They have the same saturation and brightness. There
are two tests: the D-15 and the FM-100. (The current model of
the FM-100 actually has 85 chips.) D-15 Test The D-15 hues, which are selected from all parts of the color wheel, are
provided in a box. The reference cap (blue) is fixed to the box. The
examiner removes the other caps from the box and arranges them in random
order. According to the manual, the examiner then states, “The
object of the test is to arrange the buttons according to color. Take
the button which looks most like the reference button and place it
next to it, then . . .” After the test is finished, the examiner
flips over the box and records the order of the chips. Trichromats arrange
them from 1 to 15. Deutans arrange them as follows: 1, 15, 2, 3, 14, 13, 4, 12, 5, 11, 6, 7, 10, 9, and 8 (Fig. 31); protans arrange them as 15, 1, 14, 2, 13, 12, 3, 4, 11, 10, 5, 9, 6, 8, and 7. The
examiner then connects the numbers on the score sheet in
the order in which the patient has placed them (Fig. 32).  Fig. 31. D-15 test as seen by a deuteranope (top) and by a trichromat (bottom). Fig. 31. D-15 test as seen by a deuteranope (top) and by a trichromat (bottom).
|
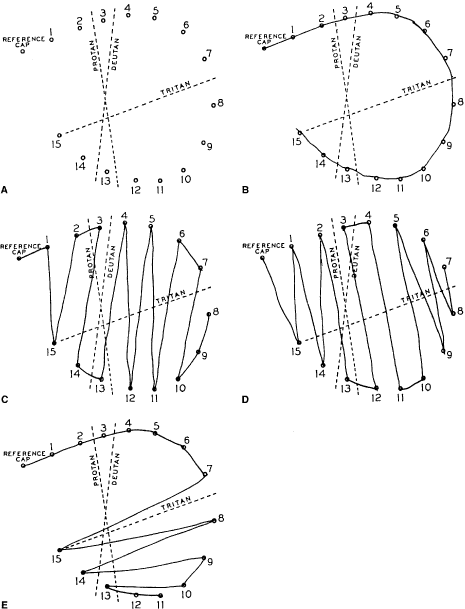 Fig. 32. A. Score sheet for the D-15 test. B. Normal trichromat. C. Deuteranope. D. Protanope. E. Tritanope. Fig. 32. A. Score sheet for the D-15 test. B. Normal trichromat. C. Deuteranope. D. Protanope. E. Tritanope.
|
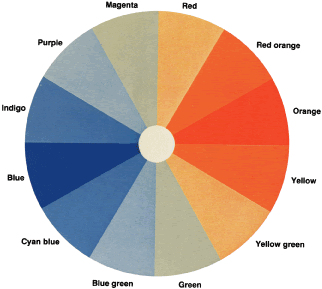
To understand why protans and deutans arrange the colors as they do and
how the D-15 so neatly separates these defects, let us return to the
color wheel and assume that we are testing a deutan (see Fig. 29). The deutan's neutral axis passes through green and magenta. Both
are seen as gray. The deutan cannot distinguish one from the other. Recall
from our discussion about the color wheel that if we add a little
yellow to green, we get yellow green; if we add a little yellow to
magenta, we get red. A trichromat can easily distinguish red from yellow
green. The deutan, however, sees green as gray. To the deutan, adding
yellow to green is the same thing as adding yellow to gray. Similarly, the
deutan sees a mixture of red purple and yellow as a mixture of
gray and yellow. Clearly, then, the deutan also cannot distinguish yellow
green from red. Both are seen as light yellow. Further, for a trichromat, yellow
green plus more yellow results in nearly pure yellow, and
red plus more yellow makes red orange. The deutan cannot distinguish
these colors either. Both are seen as a darker yellow. Let us look at the colors on the other side of the neutral axis. To a trichromat, magenta
plus blue results in purple, and green plus blue results
in blue green. To the deutan, however, both mixtures are seen as
light blue. The deutan cannot distinguish purple from blue green. For
the deutan, therefore, we can predict which hues will be confused by
drawing lines through a color wheel parallel to the neutral axis (see Fig. 29). Similar lines of confusion can be drawn for the protan and tritan. The
D-15 is a color confusion test that takes advantage of these lines. When
we plot the deutan's order of chips on the score sheet, we
see parallel lines between the colors we would have expected the deutan
to confuse (see Fig. 32). Because the protan and tritan have different neutral axes from the deutan, they
confuse different hues. For example, the protan's axis of
confusion runs parallel to the red and blue-green axis. The lines printed
on the score sheet allow us to make a quick diagnosis. The test
can, therefore, distinguish between protans, deutans, and tritans. The hues of the D-15 test were chosen so that persons with mild color defects (anomalous
trichromats) can pass. It does not distinguish between
mild and moderate color deficiency. It is felt that those who pass
the test can perform almost all functions in our society that depend on
hue discrimination. FM-100 Test In the FM-100, 85 hues, which, if arranged in a circle, would make a color
wheel, are divided into four boxes. The dominant wavelengths of box
one run from red to yellow; box two, from yellow to blue green; box
three, from blue green to purple; and box four, from purple back to red. Therefore, unlike
the D-15, which allows the subject to see colors
from 360 degrees of the color wheel at one time, the FM-100 allows the
subject to see colors from only 90 degrees at one time. The subject arranges
the caps of each box in order. After the subject has finished, the
examiner flips over each box and notes the order of placement. Please
consult the FM-100 manual for a complete explanation of the method
of scoring. Basically, the score for a cap is the sum of the differences
between the number of that cap and the numbers of the caps adjacent
to it.
| Recorded order | 5 | 6 | 7 | 8 | 13 | 11 | 9 |
| Score for the cap | | 2 | 2 | 6 | 7 | 4 | |
| How derived | | 1±1 | 1±1 | 1±5 | 5±2 | 2±2 | | The cap scores are then plotted on the score sheet (Fig. 33). Notice that 2 is the lowest possible score. However, when the total
error score is computed, 2 is subtracted from each cap score. For example, the
error score for caps 6, 7, 8, 13, and 11 (shown previously) are 0, 0, 4, 5, and 2. The
total error score is the sum of these corrected
error scores. |
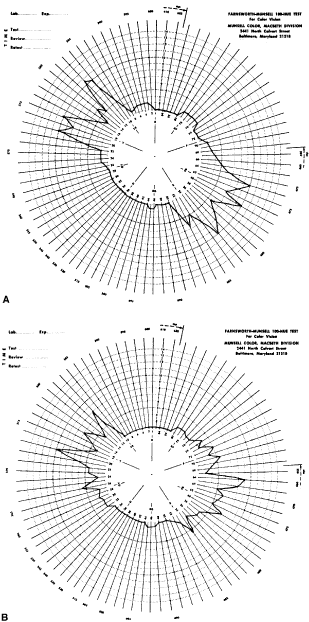 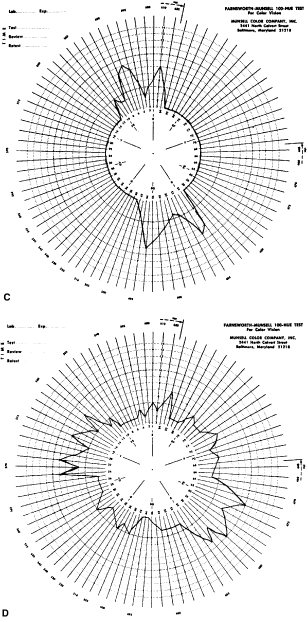 Fig. 33. Score sheet for the FM-100 test. A. Deuteranope. B. Protanope. C. Tritanope. D. Acquired red-green deficiency.
Fig. 33. Score sheet for the FM-100 test. A. Deuteranope. B. Protanope. C. Tritanope. D. Acquired red-green deficiency.
|
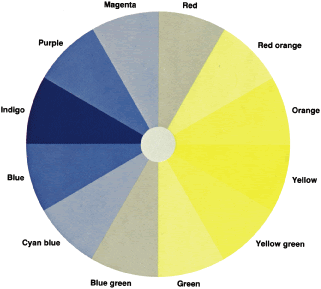
To understand why the deutan, for example, makes large errors in some parts
of the color wheel and no errors in other parts, let us return to
the color wheel as it is seen by the deutan (see Fig. 29). Near the deutan's neutral points, he or she makes few errors. The
deutan can easily distinguish blue green from yellow green. They are
seen as light blue and light yellow. On both sides of the neutral point
the chips are arranged in the order, as he or she sees it, of increasing
blueness or yellowness. Also, recall that in the D-15 test, the
test taker can select caps from all areas of the color wheel, whereas
in the FM-100 the subject can select caps from only one quarter of the
color wheel at a time. Therefore, the subject cannot make the same confusions
in the FM-100 that can be made in the D-15. Therefore, in the
FM-100 the deutan makes the largest errors 90 degrees away from his
or her neutral axis. For example, the deutan cannot distinguish red orange
from yellow because both are seen as dark yellow and cannot distinguish
cyan blue from blue purple because both are seen as dark blue. The FM-100 was designed for two purposes. The first purpose is to separate
persons with normal color vision into classes of superior, average, and
low color discrimination. A total error score of 0 to 16 indicates
superior color vision and is found in 16% of the population. A score
of 20 to 100 indicates average color vision and is found in 68% of the
population. A score greater than 100 indicates low color vision and
is found in 16% of the population. The second purpose is to measure the
zones of color confusion of persons with either congenital or acquired
color vision disorders. It is probably the best test for this purpose. Characteristic
score sheets for protans, deutans, and tritans are
shown in Figure 33. For protans, the midpoint of the zone of maximal error is between chip 62 and 70; for
deutans, between 56 and 61; and for tritans, between 46 and 52. When
a patient makes large errors in all parts of the color
wheel, he or she must have an acquired color vision disorder. KOLLNER'S RULE As a general rule, the errors made by persons with optic nerve disease
tend to resemble those made by protans and deutans, whereas those made
by persons with retinal disease resemble those made by tritans. Optic
neuritis, compression of the optic nerve, Leber's hereditary optic
atrophy, and most other optic nerve conditions affect the red-green
axis more than the blue-yellow. Autosomal dominant optic atrophy and
glaucoma are two exceptions. Fluid in or under the retina (central serous
chorioretinopathy, shallow retinal detachment, macular edema) affects
the blue-yellow (tritan) axis more than the red-green axis. On the
other hand, degenerative conditions such as cone dystrophy and Stargardt's
flavimaculatus often have a predominantly red-green defect. The
FM-100 test, discussed previously, helps to distinguish acquired
from congenital color vision disorders. |