HISTORICAL PERSPECTIVE
Fluorescein dye was initially synthesized by Adolf von Baeryer in 1871 and soon found use in the diagnosis of various corneal disorders.5–7 Later work by Paul Ehrlich in 1882 revealed the presence of intraocular fluorescein after parenteral administration in rabbits.8 Study of the fundus by angioscopy after intravenous injection of fluorescein in animals was carried out by multiple investigators until 1955, when MacClean and Maumenee used fluorescein dye in human subjects.9 Novotny and Alvis later developed a photographic system for the sequential documentation of fluorescein flow through the ocular fundus.10,11 Their published reports created a revolution in the understanding and treatment of posterior segment disease.12
CHEMICAL AND PHYSICAL PROPERTIES
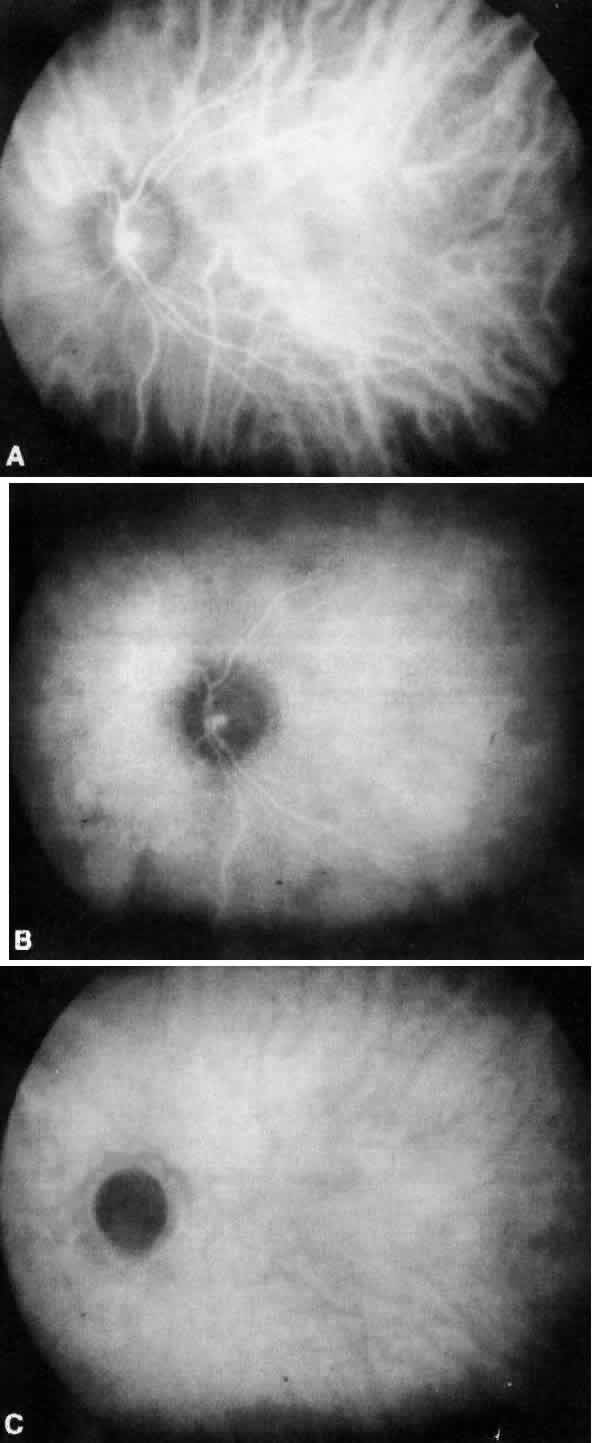
Sodium fluorescein (C20H10Na2) is a highly water-soluble complex organic molecule with a molecular weight of 376.27.6,13,14 Its clinical utility results from the physical property of luminescence—the emission of light by means other than incandescence. In the case of fluorescent material, the emission of light is a direct result of excitation by light of a shorter wavelength and higher energy level. Unlike phosphorescent material, the rate of light emission is so rapid as to appear instantaneous. Quantum theory states that the absorption and emission frequencies for a particular material are spectrum specific for that particular molecule (Fig. 1). Light energy is released when an excited fluorescent material spontaneously decays from a higher energy state to a lower level.
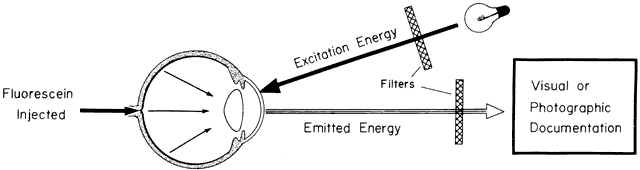
Excitation of fluorescein occurs when it is exposed to blue wavelengths between 465 and 490 nm, resulting in emission of yellow-green frequencies (520 to 530 nm).13–17 In the bloodstream, fluorescein is excited by a wavelength of 465 nm and emits a wavelength of 525 nm.17,18 This greater spread between excitation and emission frequencies is clinically helpful in their separation by photographic filters during angiography (Fig. 2; see Fig. 1).17,18
Sodium fluorescein has several physical and chemical properties that make it an excellent diagnostic tool. It is an inexpensive, water-soluble and relatively inert organic plant resin that exhibits its maximum fluorescence at a near normal blood pH of 7.4.6,13,14,16 Its excitation and emission wavelengths are in the visible spectrum, allowing use of standard photographic equipment and materials. In addition, the molecular size of fluorescein prevents its passage through the tight endothelial junctions of retinal blood vessels and zonula occludens in retinal pigment epithelium while allowing for rapid diffusion in fluid compartments.13,14,16 Conversely, sodium fluorescein's high percentage of binding to albumin and other bloodborne proteins drastically reduces the amount of free fluorescein readily available for excitation.14
ADMINISTRATION
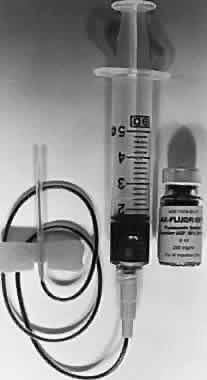
Five milliliters of 10% sodium fluorescein solution is routinely given intravenously at the start of the procedure. A 25% solution is also available and may be better tolerated by the patient. Evaluation of smaller, 2-ml injections of 10% solution by Nasrallah and associates have given equally satisfactory results with fewer side effects.19 A scalp needle attached to a small syringe is ideal for administration (Fig. 3). The angiographic study is initiated with the patient seated comfortably at the fundus camera. The fluorescein dye is injected rapidly into an antecubital vein. Blood should be refluxed into the fluorescein-containing syringe before injection to ensure entry into the vein. Administration is monitored carefully because extravasation of dye results in severe localized pain and even overlying skin necrosis.20,21 Blood precautions should be observed with discarded needles and syringes, and gloves should be worn by those administering dye.
|
Within 3 to 5 minutes, the fluorescein is distributed equally throughout the blood. It is rapidly eliminated, predominantly by the kidneys14,16,22; most of it is removed from the bloodstream within 1 hour. Patients should be alerted to expected changes in skin tone and urine color in the post-test period.
Oral administration of fluorescein is also possible in those individuals for whom intravenous injection is difficult or impossible. Oral administration allows assessment of the blood-retinal barrier, but it is inadequate in determining blood flow velocities or anatomic detail.23–25 Ten percent sodium fluorescein is mixed with orange juice to give a 1% to 2% solution; 1 g of dye is given to patients weighing 50 kg or less. More dye, up to a maximum of 2 g, is given to heavier patients.23 Serum concentrations of orally administered fluorescein peak at 30 minutes and remain relatively stable for 1 to 2 hours at levels similar to intravenously administered dye.23 Although the reported incidence of side effects is reduced with this route, allergic reactions still may occur.26
TOXICITY
Although numerous reports of side effects secondary to fluorescein administration are present in the literature, it remains a remarkably safe diagnostic compound. Nausea and emesis are the most commonly reported reactions, occurring with a frequency of 5% or less.27 Syncope, thrombophlebitis, temperature elevation, nerve palsy, and local tissue necrosis are classified as moderate adverse reactions whose incidence is less than 1%.20,26,27 Severe adverse reactions requiring aggressive intervention include laryngeal edema, bronchospasm, anaphylaxis, circulatory shock, and myocardial infarction. A survey by Yannuzzi and associates reported one related death in more than 220,000 angiographic studies of the ocular fundus.27 With patients in whom toxicity is suspected, an intradermal skin test has been recommended.25 An emergency tray and oxygen should be available whenever fluorescein is administered. Prophylaxis for possible adverse reactions is controversial. Although there have been no reports of human fetal complications secondary to fluorescein injection during pregnancy, many physicians avoid administration if possible in pregnant individuals.27,28
ANATOMIC CONSIDERATIONS
Fluorescein angiography of the iris and fundus is possible because of the unique specialization of the eye as an optical instrument. Only in the eye is the vascular system open to close scrutiny through noninvasive means.
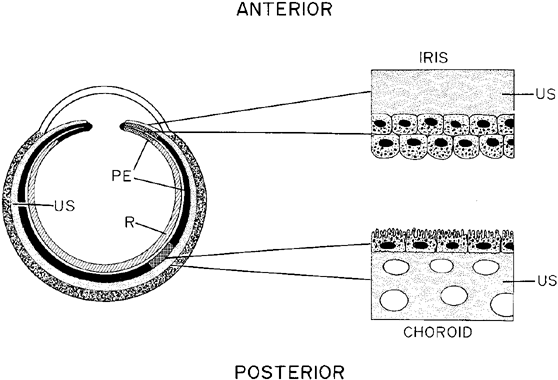
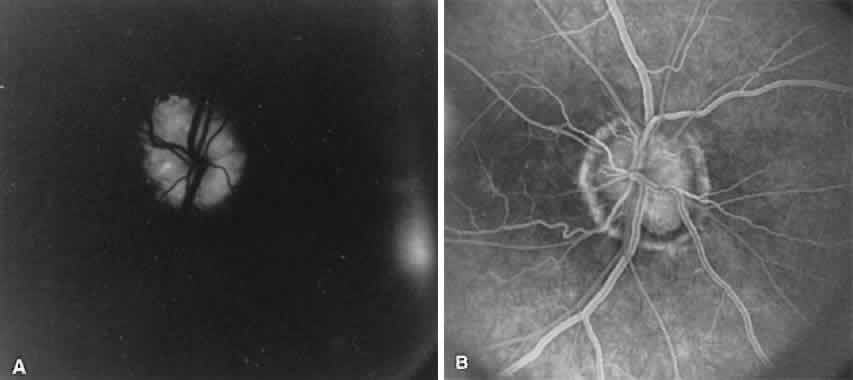
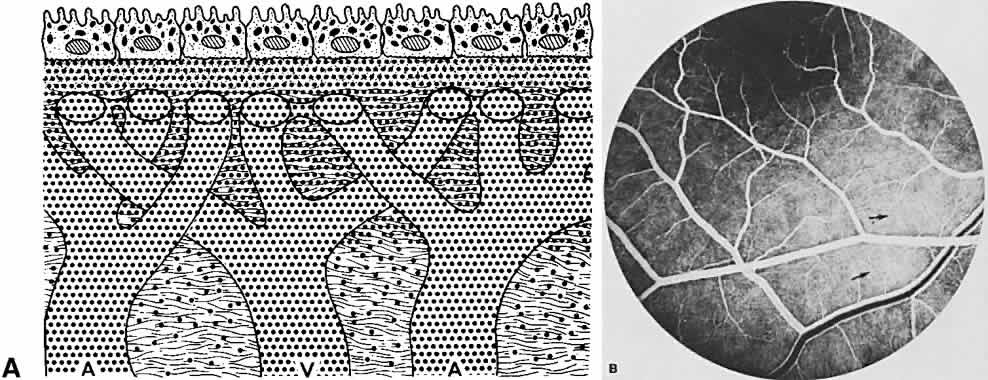
The ocular fundus has two distinctly separate vascular systems—retinal and choroidal—separated by a specialized pigmented monolayer, the retinal pigment epithelium (RPE). Embryologically, the sensory retina and RPE are derived from the inner and outer layers of the optic cup. The choroid and its vasculature lie posterior to the RPE. The fluorescein angiographic patterns of the posterior uvea are, therefore, always partially obscured by the RPE (Fig. 4). The degree of pigmentation and the pathologic changes in this pigmented layer markedly influence the choroidal angiographic appearance. In the interpretation of fundus fluorescein angiograms, the physician must keep in mind and be familiar with the anatomy and interaction of these three layers.
CHOROID
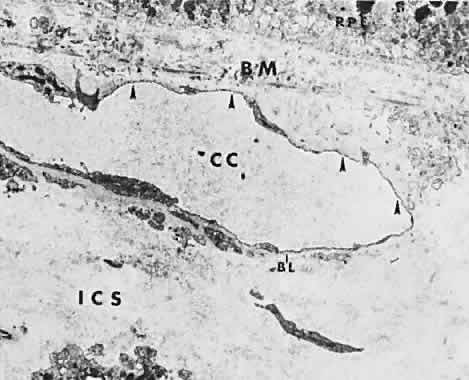
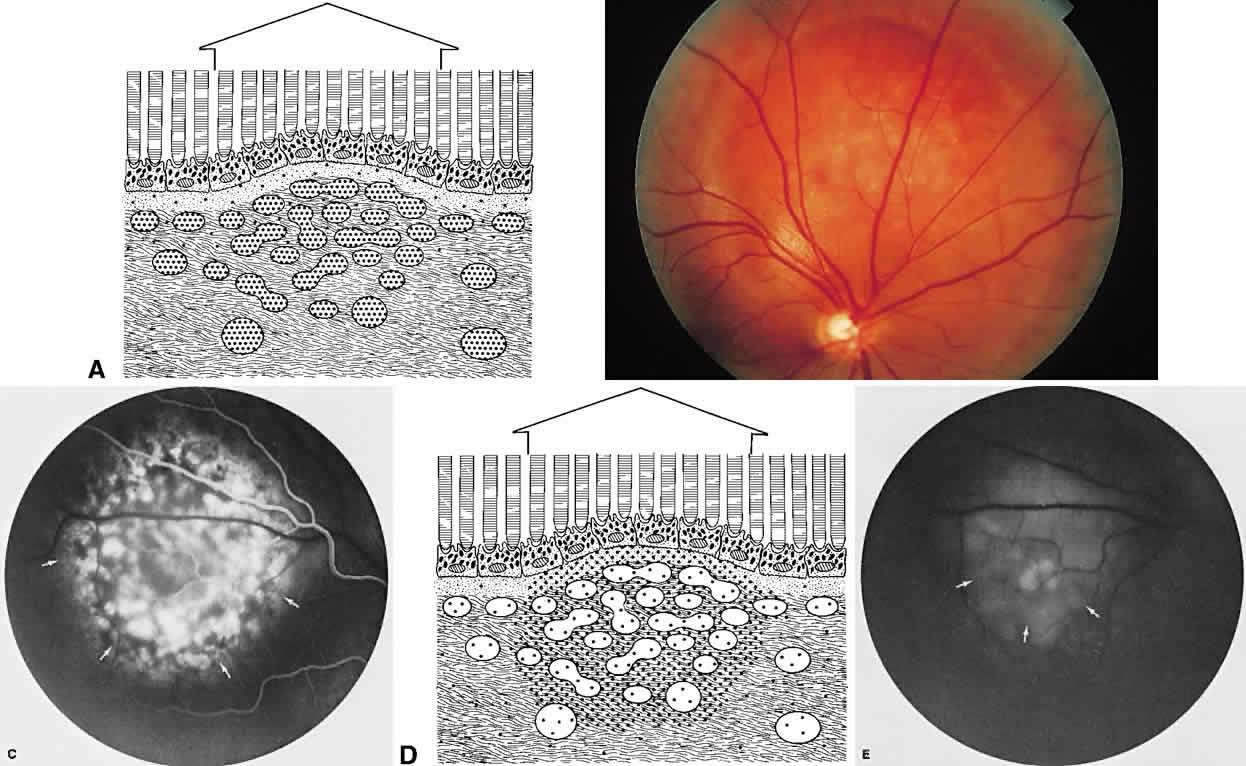
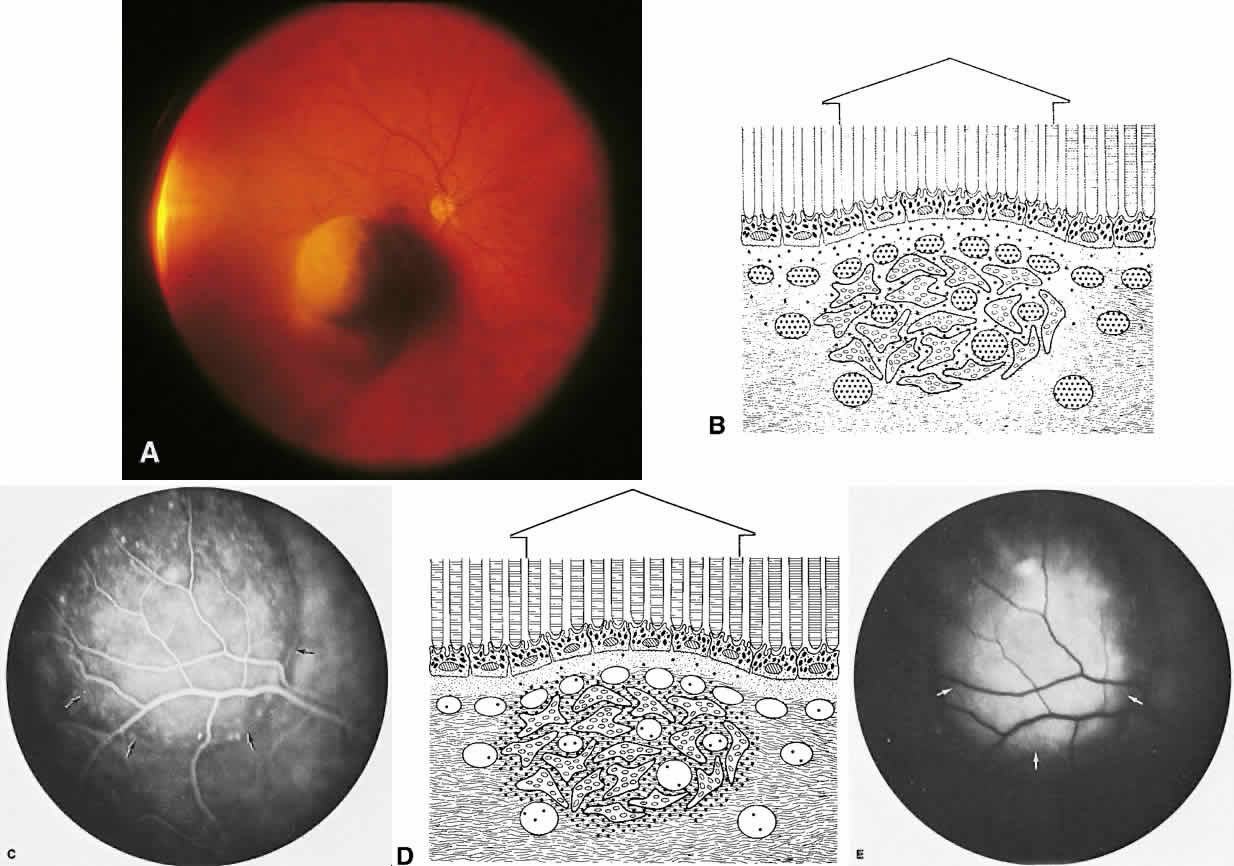
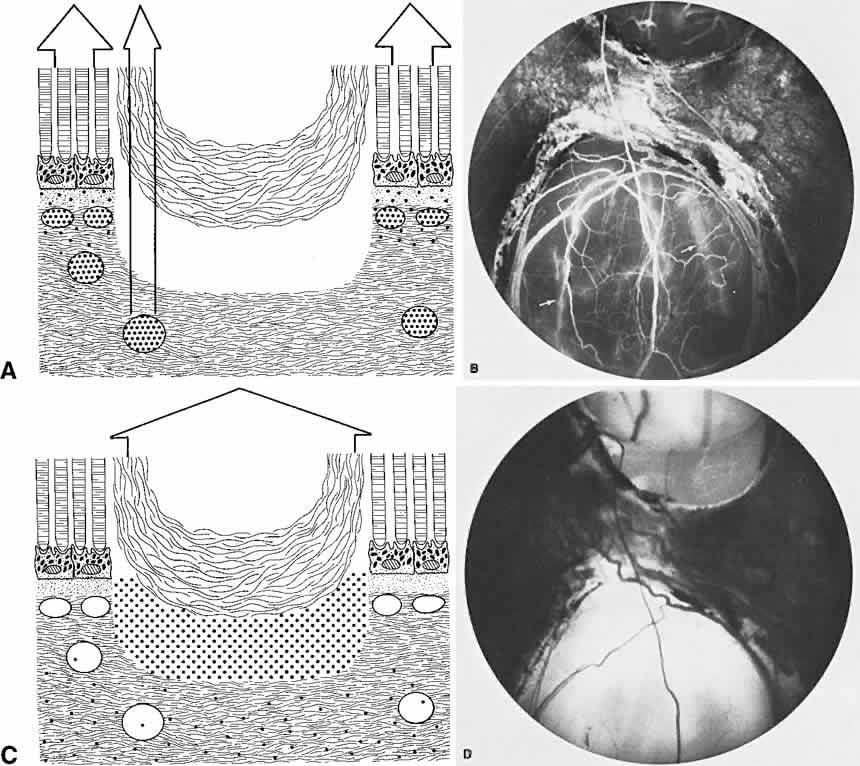
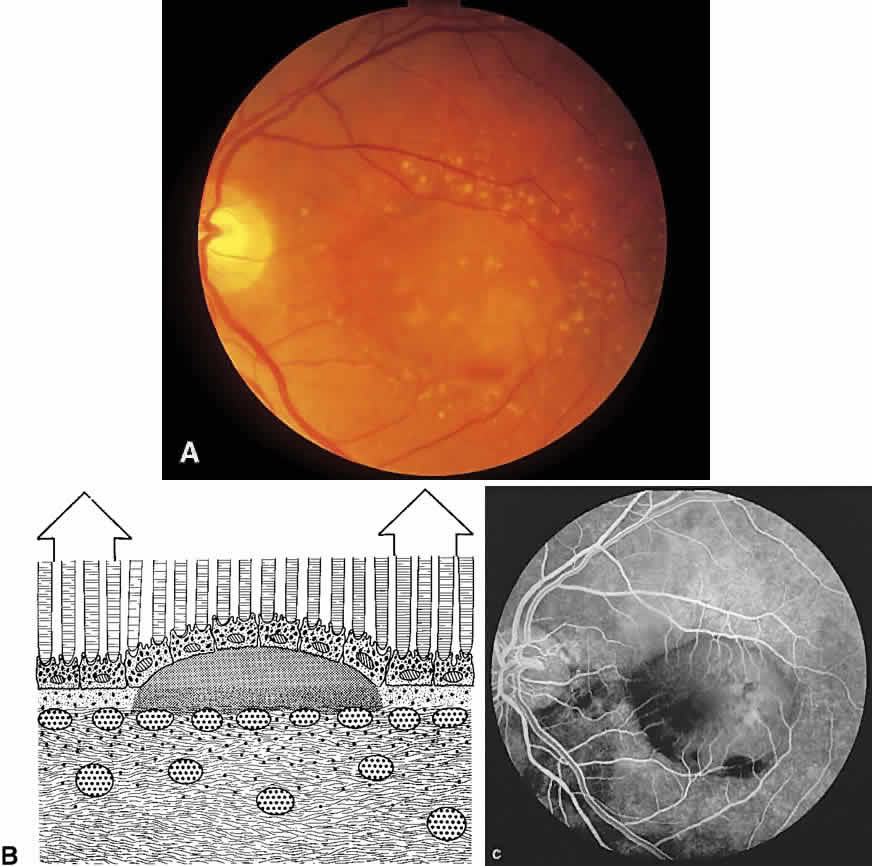
The choroid is a highly vascular connective tissue layer with an average width of 0.25 mm. It is made up of three distinct layers and is perfused by the long posterior, short posterior, and recurrent anterior ciliary arteries. Drainage is primarily through the vortex veins. Its capillary system, the choriocapillaris, is innermost and lies directly beneath Bruch's membrane. The choriocapillaris has a lobular pattern made up of a central arteriole, peripheral draining venules, and an intervening capillary bed. The capillary walls are extremely thin and contain multiple fenestrations, allowing a high degree of fluid transport (Fig. 5). These fenestrations are thought to be actual openings in the endothelial wall through which fluid can undergo passive exchange between the lumen of the vessel and the surrounding extravascular space. As fluorescein enters the choriocapillaris, it quickly passes into the extracellular space. The outer choroidal layers are nonfenestrated and, similar to other typical arteries and veins, do not normally leak fluorescein dye.
Under normal conditions, the choroid begins to fill with fluorescein a moment prior to the retinal vascular system. This filling is difficult to study by fluorescein angiography because of obscuration by the overlying retina and RPE, its rapid filling, and leakage of fluorescein from the choriocapillaris.6
RETINAL PIGMENT EPITHELIUM
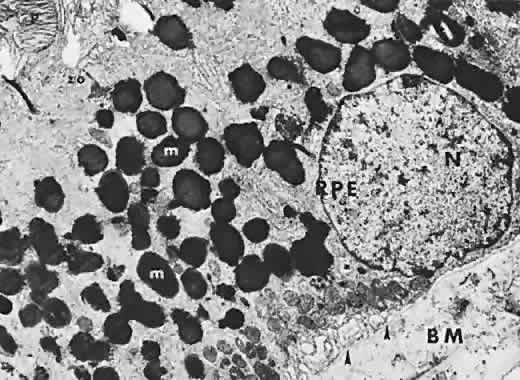
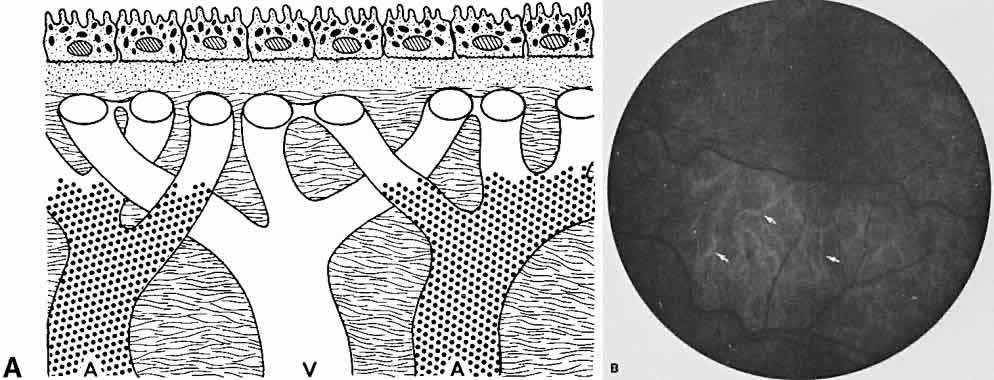
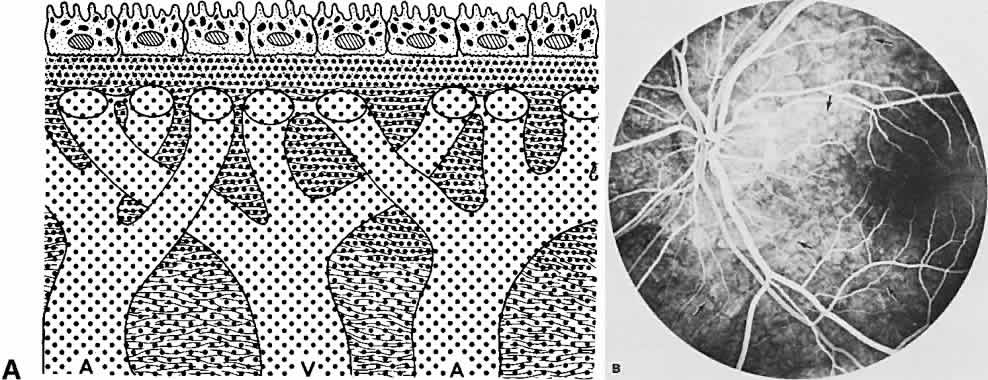
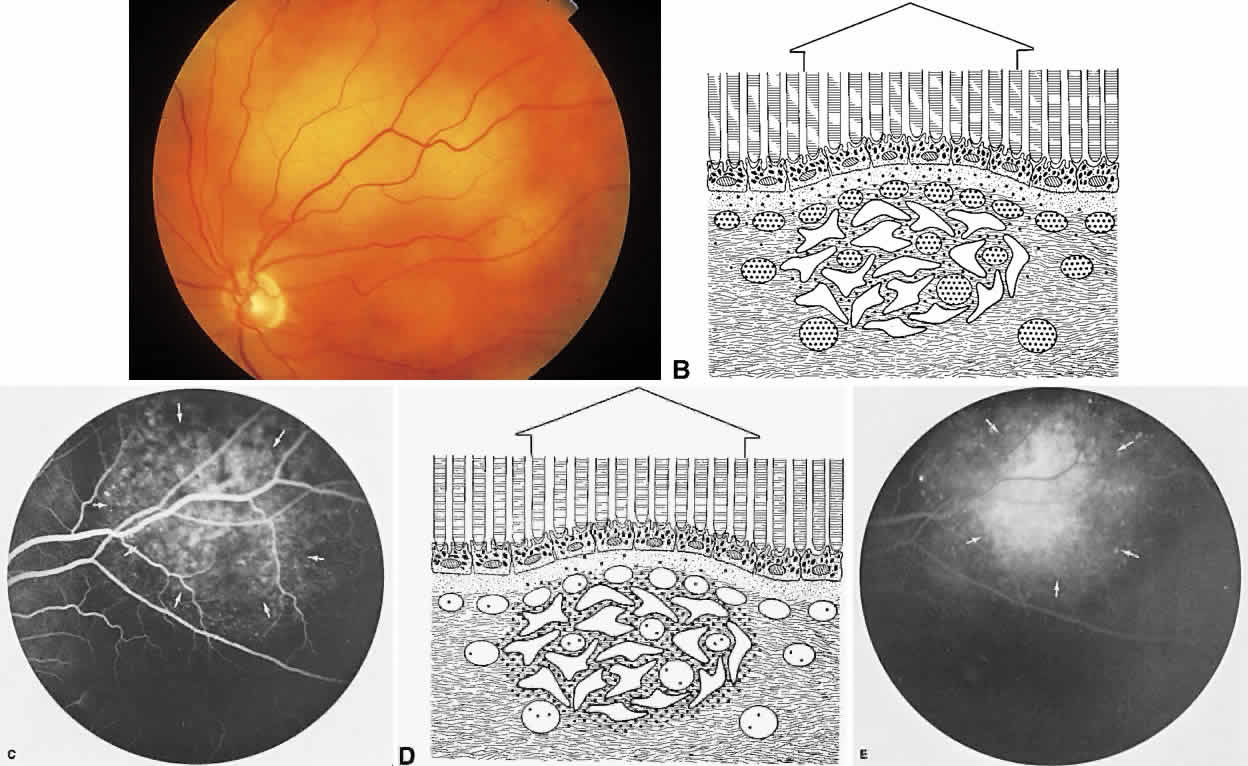
The RPE is a specialized monolayer of pigmented epithelial cells separating the choroid and sensory retina. It functions physiologically as both a metabolic support platform for the overlying photoreceptors and as an effective barrier against passive molecular transport between the choroid and retina.29,30 In its normal state, the RPE prevents leakage or transport of fluorescein into or out of the choroidal space.
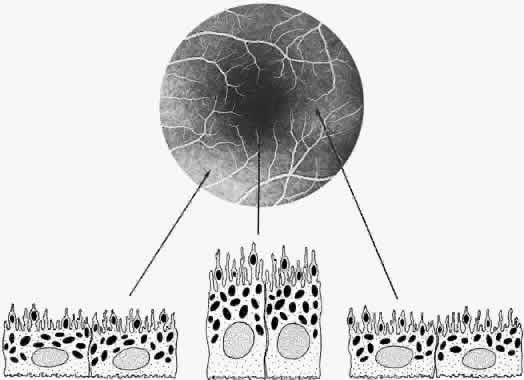
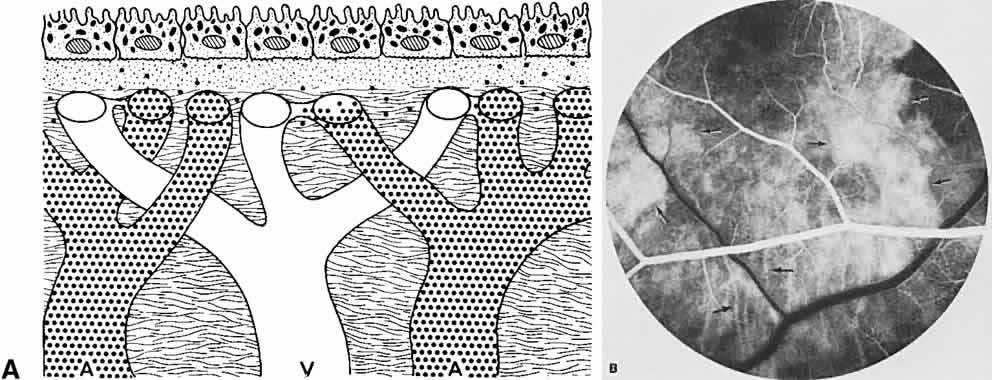
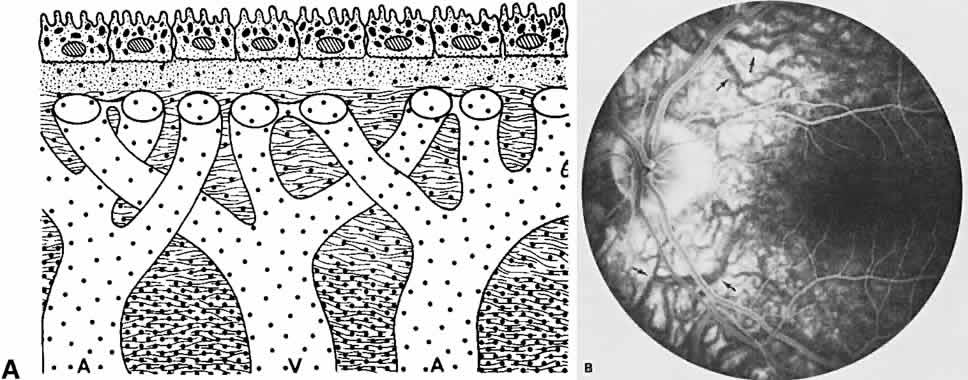
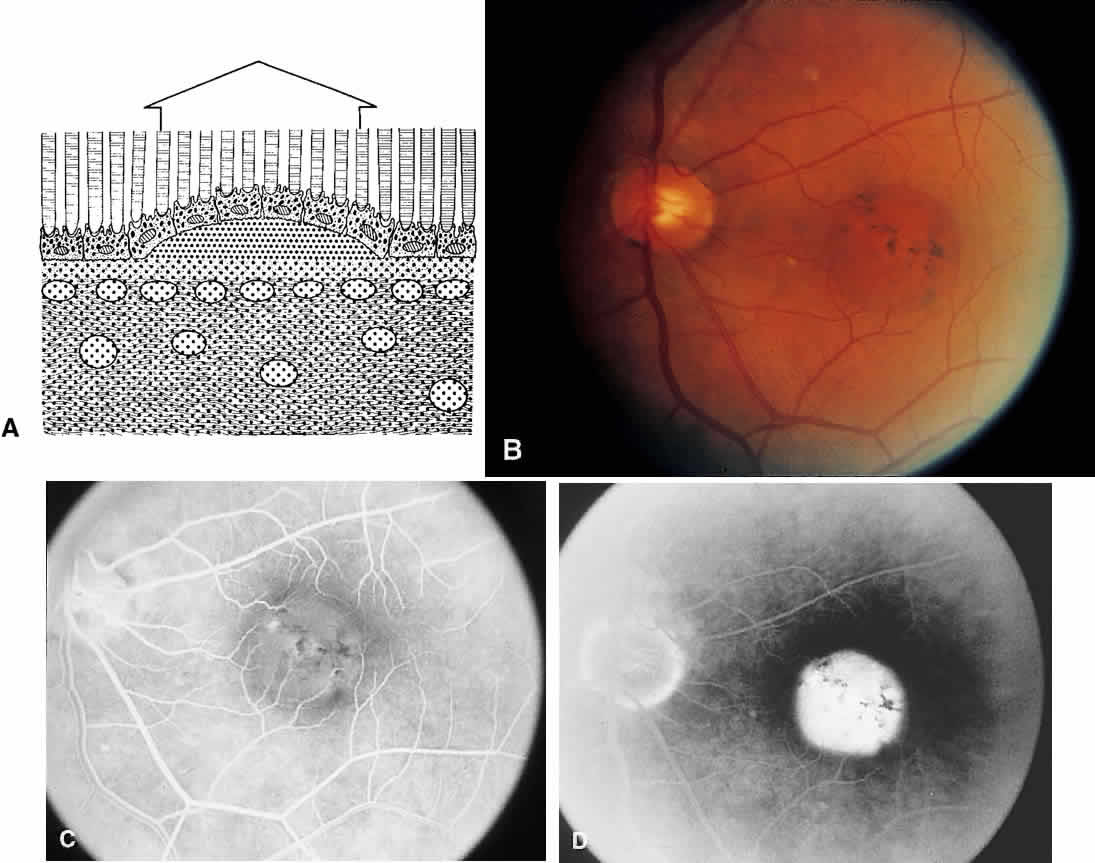
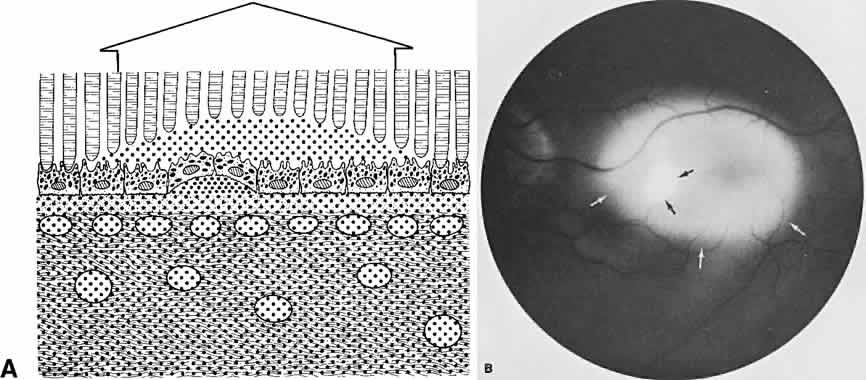
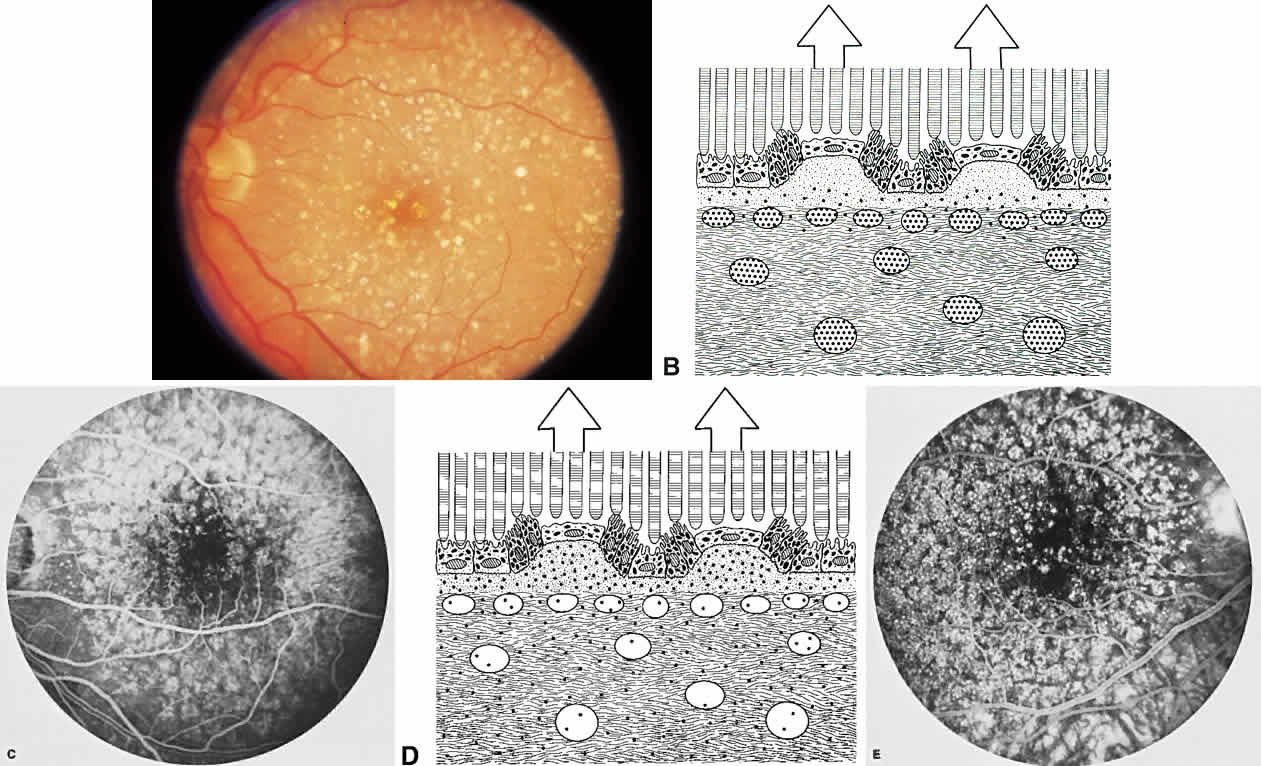
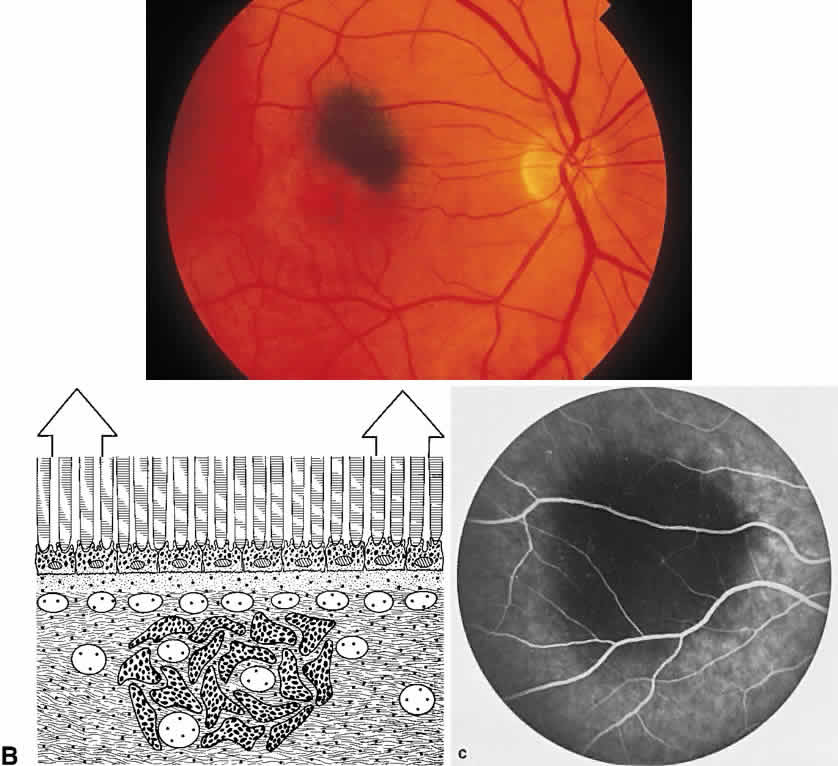
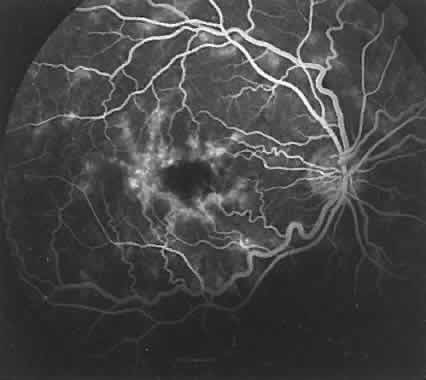
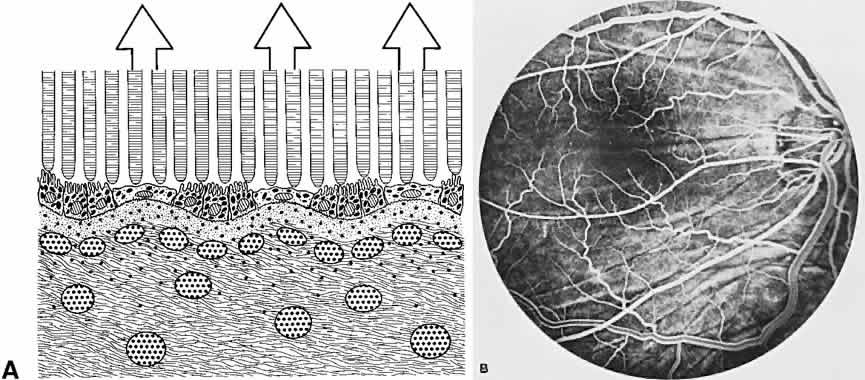
The RPE also functions as an optical barrier because of the presence of pigmented melanosomes (Fig. 6). The density of this pigment varies with retinal location; it is greatest in the foveomacular region and least anterior to the equator. There are more pigmented epithelial cells per unit area in the foveomacular region than in the periphery. Posteriorly, the cells are tall and columnar, with several layers of melanosomes crowded together. Anteriorly, the cells gradually become flatter and more cuboidal, with a corresponding loss of relative pigment concentration (Fig. 7). In any normal eye, regardless of the comparative fundus color, the greater pigmentation in the foveomacular area gives this zone a darker clinical appearance when compared with the remainder of the posterior pole and periphery.
RETINA
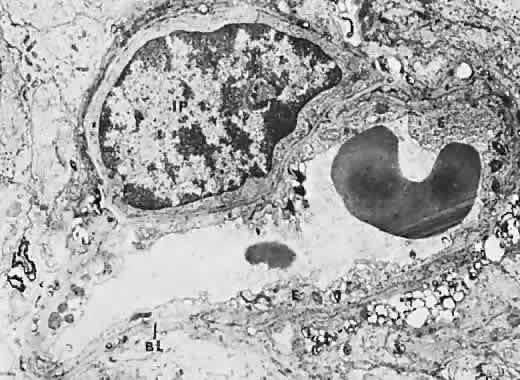
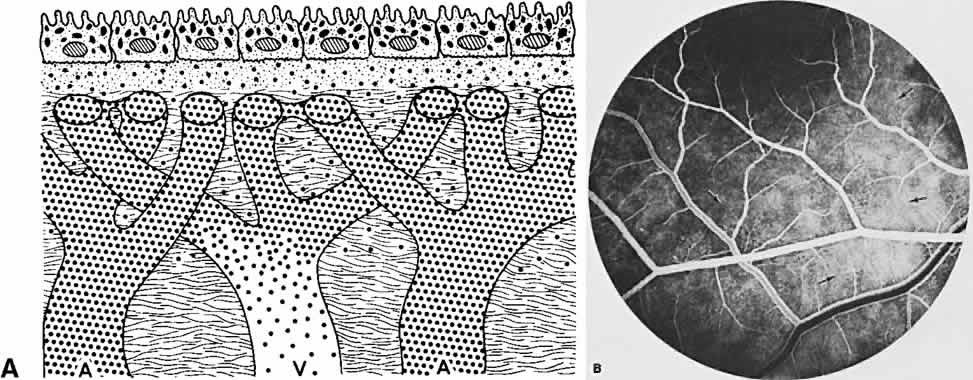
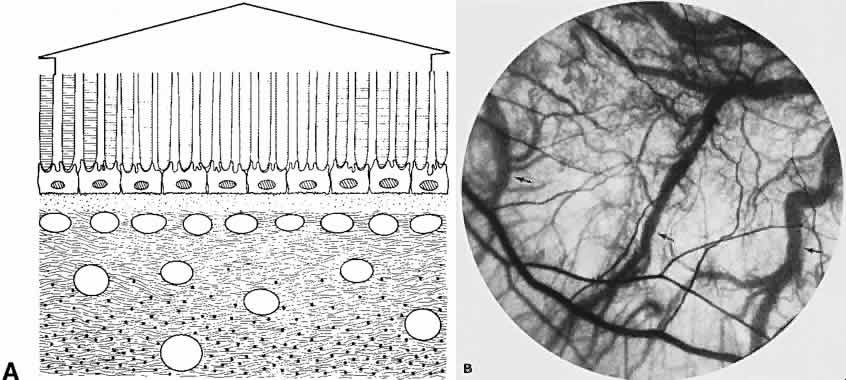
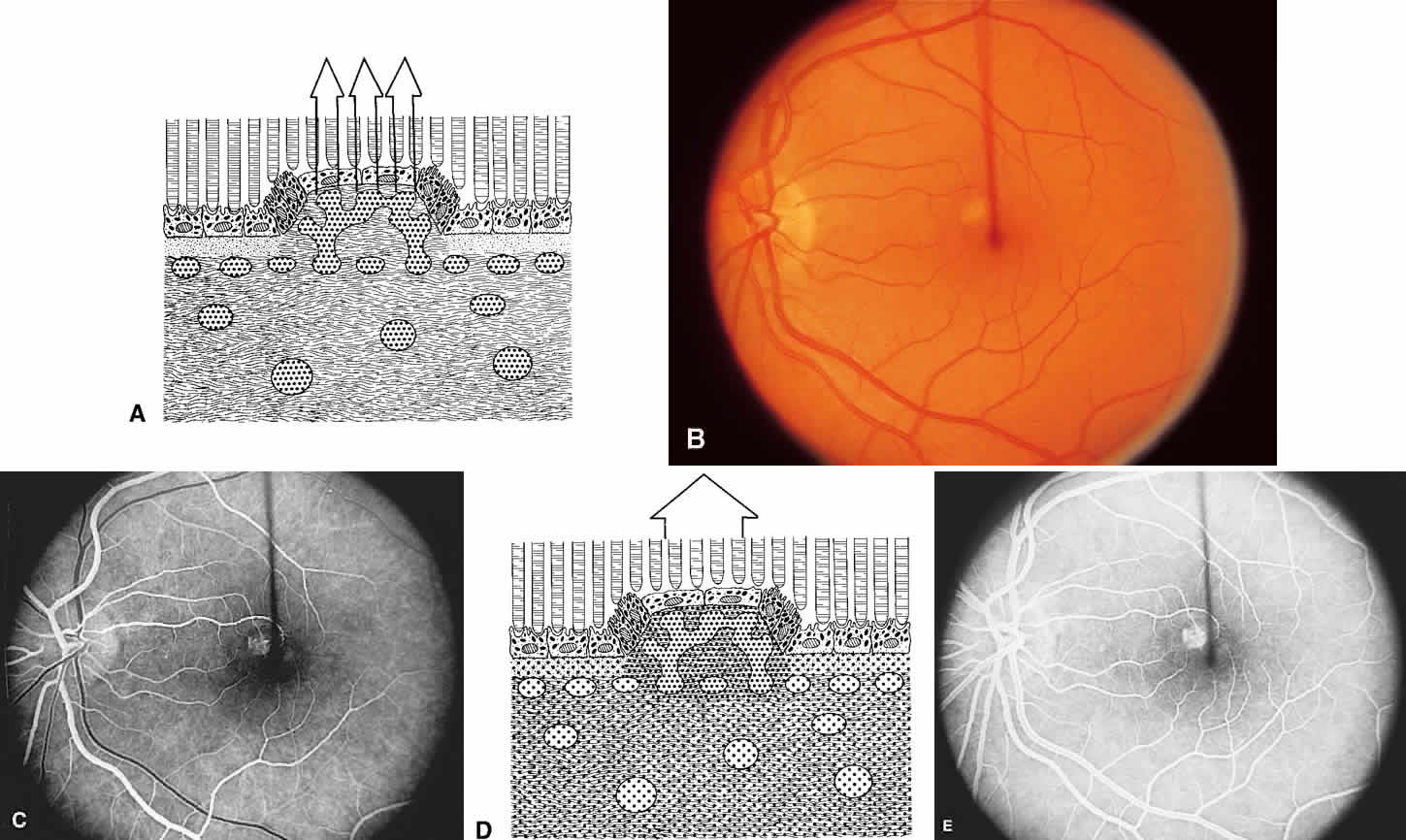
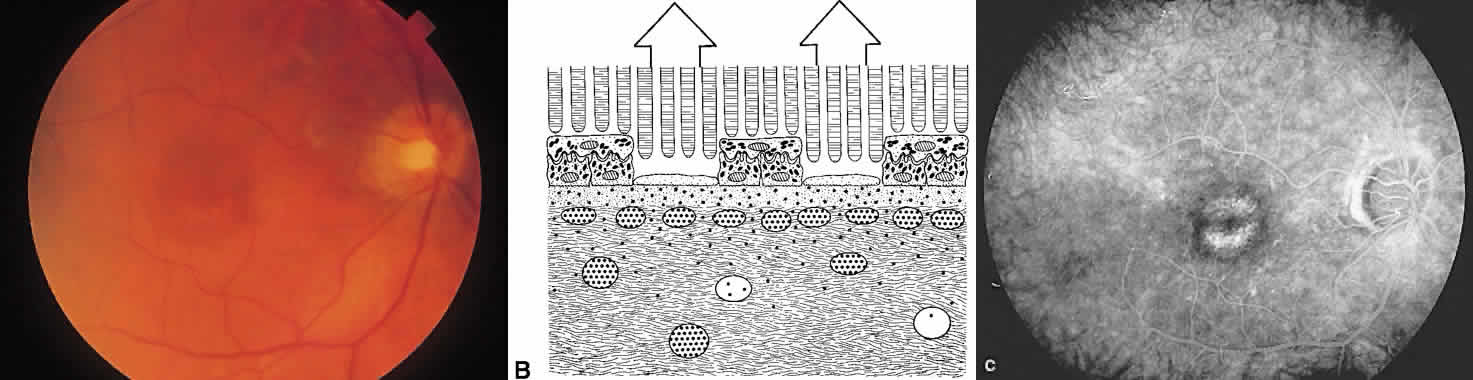
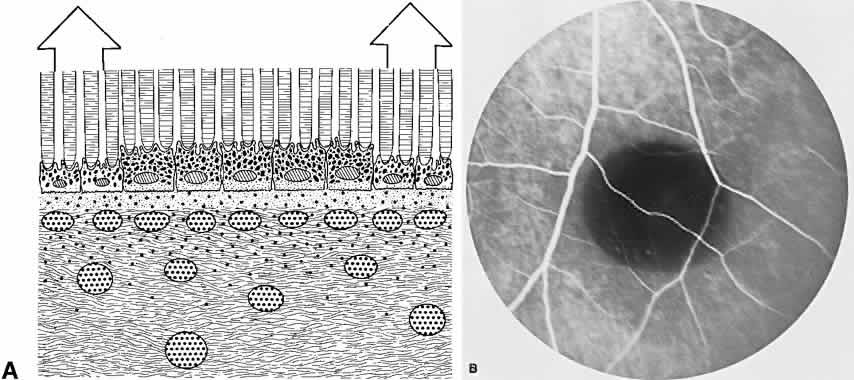
The retina, except for its vasculature, may be regarded as a transparent tissue comprised of tightly packed layers of cells with essentially no extracellular space. The retina is perfused by the central retinal artery and cilioretinal vessels present in 32% of eyes.31 The capillary bed is nonfenestrated and fluorescein does not, therefore, leak from the retinal vessels into the surrounding tissue (Fig. 8). Functionally, the retinal vasculature represents a “closed” system, as opposed to the choroid, in which fluorescein is free to move between the intravascular and extravascular compartments. The retinal circulation normally fills with fluorescein after the choroidal flush; however, a cilioretinal artery fills at the same time as the choroid, preceding the rest of the retinal circulation.
The retina also serves as an optical filter because of its high content of xanthophyll pigment, especially in the macular region. This yellow pigment has its highest concentration in the outer nuclear and plexiform layers, and may selectively absorb blue excitation frequencies.16
IRIS
The iris stroma, like the choroid, is composed of vascular connective tissue. Although electron microscopy and tracer studies have demonstrated the absence of fenestrations in both animal and human eyes,32–34 other reports have supported the presence of gaps in the junctions between endothelial cells in some animal iris tissue.34–36