
|
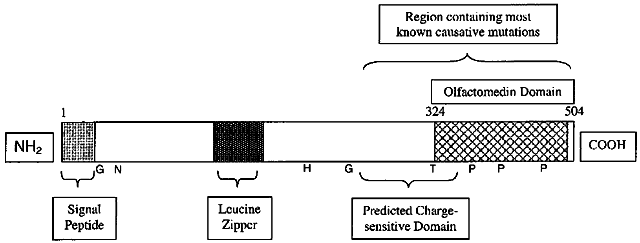
| Fig. 1. Schematic of the human myocilin protein. The amino terminus contains a signal peptide plus a leucine zipper, while the carboxy terminus contains an olfactomedin-like domain where many mutations are found. The leucine zipper sequence may be important in protein-protein interactions. Numbers represent amino acid position. NH2 marks the amino terminus. COOH marks the carboxy terminus. Additional points on the protein predicted from the sequence by Nguyen and colleagues are two glycosaminoglycan initiation sites (G), an N-glycosylation site (N) and a hyaluronic acid binding site (H).52,53 T indicates the position of the Glu323Lys mutation that causes a change in the translocational pause of TIGR protein biogenesis.143 P marks conserved sequences at which phosphorylation is predicted to occur and C marks a predicted C-terminal peroxisomal targeting sequence.47 The charge-sensitive domain was predicted by Shimizu and colleagues.49 |