There are three approaches to laser cyclodestruction. The first of these is transpupillary focal photocoagulation of the ciliary processes.14 In many eyes with neovascular glaucoma, the iris is pulled forward sufficiently such that the ciliary processes are visible with a gonioscopy lens. The same may be true in some eyes with widely dilated pupils or in eyes with large iridectomies. In these eyes, the anterior face of the ciliary processes can be photocoagulated with an argon laser. The laser is set at 1000 mW for 0.2 seconds using a 100-μm spot, and as many processes as are visible are treated. Because the more posterior aspects of the processes are not visible and therefore not treated, the results of this treatment are very unpredictable and the eye may have to be treated several times. This technique is not very widely used.
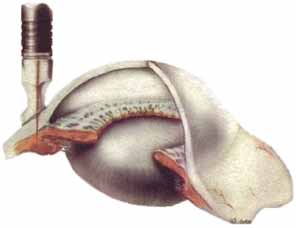
The second approach is transscleral laser cyclotherapy. In this technique laser energy is pulsed through the sclera to the ciliary body where the laser energy is absorbed by the melanin and converted into heat that damages the ciliary processes. At present, two different types of lasers are being used for this treatment: diode lasers and Nd:YAG lasers. Our experience and that of others has been that these two types of lasers are quite similar in both efficacy and side effects.15,16 In the beginning, the Nd:YAG lasers were used in a noncontact mode where the aiming beam was focused on the conjunctiva, but the laser energy was retrofocused deeper into the eye. Then a fiber-optic delivery system was developed with a synthetic sapphire tip that was placed on the eye that allowed contact administration of the laser energy. Similarly, a delivery system for the diode laser was developed with a special handle known as the G-probe that was especially designed for placement of the probe at the limbus (Fig. 1).
The Nd:YAG laser uses slightly higher energy (6 to 8 W), shorter duration (0.7 second) pulses. Approximately 32 laser applications are evenly spaced around the 360 degrees of the limbus avoiding the 3 and 9 o'clock meridia. The tip of the probe is centered approximately 1 mm behind the limbus. After the treatment, dexamethasone solution is injected subconjunctivally and the eye is patched until bed time. Glaucoma medications are continued unchanged and topical corticosteroids are begun the next day. My patients rarely experience significant posttreatment pain, and I do not routinely prescribe an analgesic. Also, unlike others, I do not routinely prescribe cycloplegics. The patient is seen approximately a week later and the glaucoma medication is adjusted depending on the IOP response to the treatment. The topical corticosteroids are tapered based on the inflammatory response. In my experience, a majority of the eyes treated will experience a rebound in the IOP over weeks to months and the treatment will have to be repeated.
The treatment protocol with the diode laser is similar with some minor differences. The laser applications are longer in duration (1.5 to 2.5 seconds) but use lower energy (1.5 to 2.25 W) and an average of 25 applications are placed. The G-probe is placed with the foot plate at the limbus. The postoperative management is the same as with the Nd:YAG laser.
The third approach to laser cyclotherapy is focal photocoagulation of the ciliary processes using an endoprobe. The endoprobe contains one fiber-optic pipe that allows visualization of the tissues and a second one to deliver the diode laser energy. In general, there are two approaches that can be used. In the first approach, the endoprobe is entered into the anterior chamber via a limbal incision and then passed through the pupil behind the iris until the ciliary processes are visualized and then photocoagulated.17 This approach is particularly used in combination with phacoemulsification and posterior chamber intraocular lens (PC IOL) insertion. The second approach is posteriorly through the pars plana and is usually performed as part of a vitrectomy.18 The laser energy is adjusted to cause blanching and shrinkage of the processes but no vaporization of tissue. Usually approximately 180 degrees of the ciliary body is treated.
In the past, cyclodestructive procedures have been reserved for end-stage glaucoma eyes after everything else has failed. Recently, several reports have been published from Third World countries where is has been used earlier in the disease process.19 With short to medium follow-up, the results have suggested that it may be a reasonable alternative to trabeculectomy. Several of the early series of laser cyclotherapy cases with medium to long-term follow-up reported unexplained decreases in visual acuity and suggested that this was a side-effect of the laser treatment.20 Long-term follow-up of trabeculectomy eyes in the AGIS study and other case series have shown that some of these eyes have similar decreases in visual acuity.6,19 Thus, there is a growing question as to whether this side effect is specific to the laser therapy. I have performed laser cyclotherapy on patients with good visual acuity (20/80 or better) and the majority have maintained this vision after up to 6 years of follow-up.21 A second concern with laser cyclotherapy is the risk of sympathetic ophthalmia. Several cases of sympathetic ophthalmia in fellow eyes were reported following noncontact Nd:YAG laser cyclotherapy when this technique was first used,22 but no additional reports have appeared in spite of the great increase in laser cyclotherapy.