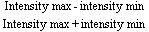THE CONCEPT OF SPATIAL FREQUENCY
The visual image can be thought of as being made up of pixels (picture elements) much like a computer screen. The finer the pixel size, the better the resolution. For example, look at the nude in Figure 1. Note that the finer the pixel system, the more information is presented. For a black-and-white picture each pixel also contains shades of gray. Thus the pixel system can present different levels of contrast. If the retina performs as a pixel system, how might its information be transmitted to the brain? One popular theory suggests that it is as if a number of different wire services record the retinal picture and then file their story back to the brain. One wire service only has a resolution equivalent to 20/200 and thus only has a large pixel system. You might say that this wire service can only see the big picture. The next wire service has a higher resolution system and reports more detail, with smaller pixels. Still another wire service reports on even finer details and has a 20/20 size pixel. With such a series of wire services, the transmission system must be compatible with a memory filing system, each wire service memo ultimately funneled into memory. In such a system, memory can fill in unreported details when the retinal image is not clear. For example, when life scenes are blurry from fog, snow, rain, or dust, only the large pixel system would carry information. The memory filing system would then be able to add details not actually seen. Such a system of a series of vision channels has another advantage. Even if a few of the nerve channels have been damaged or diseased, some information about every scene has a chance of being registered. Furthermore, if there is a disease or injury to an optical element of the eye (cornea or lens) a fuzzy picture devoid of details gets through the larger pixel channel, and the memory file improves the overall perception of the image. It has even been suggested that in young patients with eye disease, a contrast enhancement mechanism comes into play that can push up sensitivity in the channels that are working.1
Sine Waves
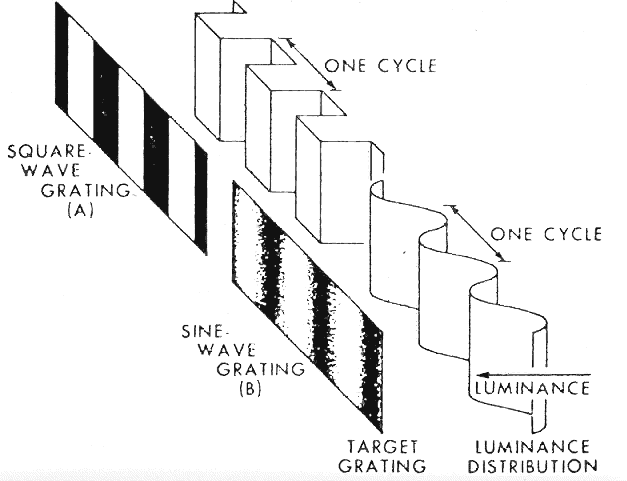
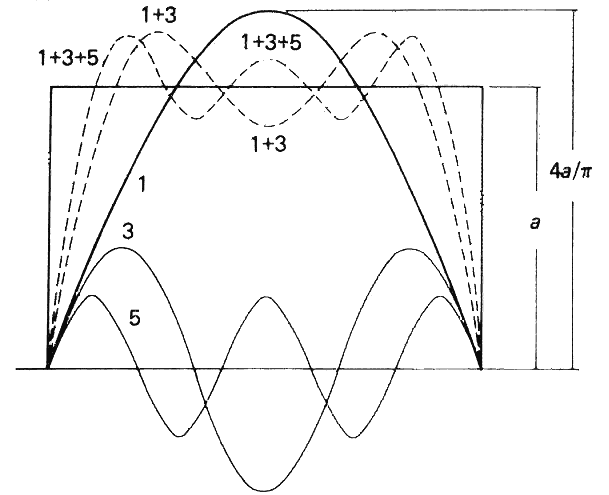
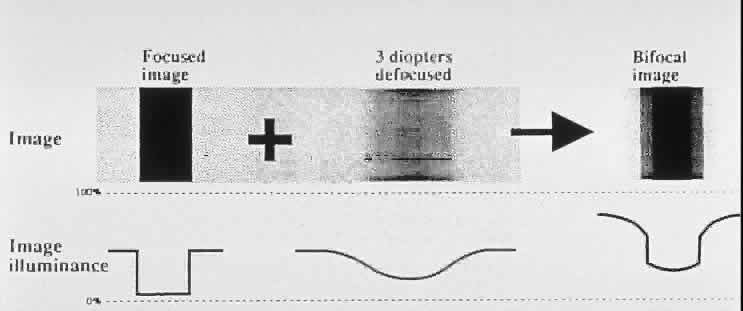
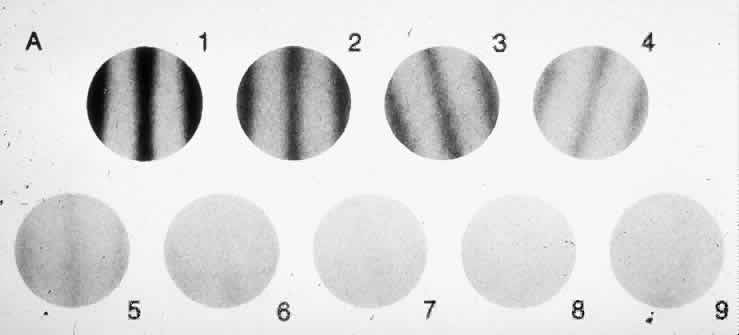
The letter E on the Snellen chart can be considered to be made up of dark horizontal bars against a white background. The spacing between the bars can be described in terms of angular subtense. The visual psychologist would describe the target as a pattern of square waves with a certain spatial frequency (i.e., the 20/20 letter has a spatial frequency of 30 cycles per degree; Fig. 2). Thus spatial frequency refers to the number of black-and-white bars (1 cycle) within a degree of angular subtense. However in optics, very few images can be described as perfect square waves with perfectly sharp edges. Diffraction, chromatic aberration, spherical aberration, and oblique astigmatism tend to make most edges fuzzy. Therefore, if one plotted the light intensity across the retinal image of a black bar against a white background, one would get a modified sine wave pattern. Sine wave patterns have great appeal to the mathematician because they can be combined to produce any desired pattern. In Figure 3 a combination of different sine waves are shown adding up to produce a new sine wave pattern. This trick of being able to break down any alternating pattern (be it an electrocardiogram or a trumpet sound wave) into a unique sum of sine waves is known as a Fourier transformation. A Fourier transformation is based on the assumption that any wave may be described as a sum of sine waves that has different spatial frequencies, amplitudes, and phases.
|
|
The visual scientist also feels that the visual system operates by breaking down observed patterns and scenes into sine waves of different frequencies. Present theory suggests that a few thousand patches of the visual image are analyzed into about six different spatial frequencies at around 20 different orientations.2 The brain then adds up all the information to produce the mental impression of a complete picture. Fourier transformations are the way that the visual system encodes and transmits and reconstructs retinal images. Experimental evidence in cats shows that the different channels that exist in the retina, lateral geniculate body, and cortex selectively carry appropriate information related to different spatial frequencies.3 It has also been shown that all channels respond to contrast with the cortex actually showing a linear relationship between the amplitude of the neuronal discharge and the logarithm of the contrast of the sinusoidal grating.
Contrast Sensitivity of Different Spatial Frequencies
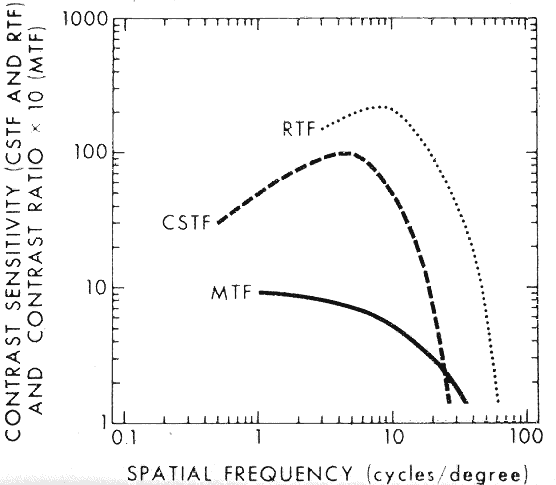
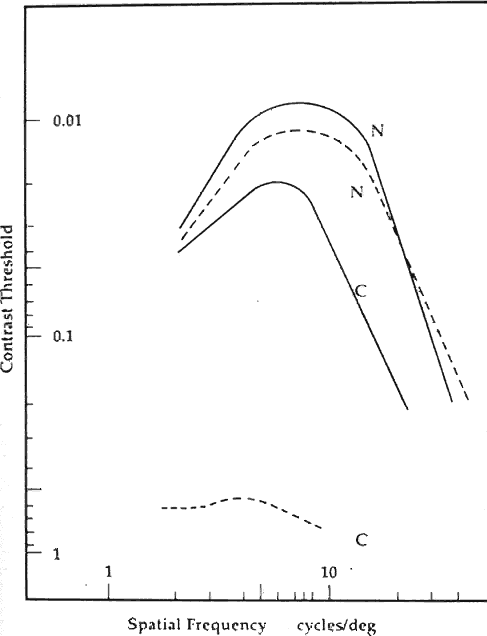
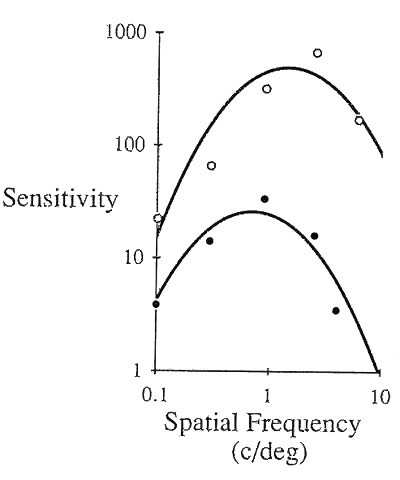
One might intuitively think that an optimal visual system would have similar contrast sensitivity thresholds for all the different spatial frequencies. Interestingly, all good optical systems have a better contrast sensitivity for lower spatial frequencies. The contrast sensitivity gradually gets poorer for higher spatial frequencies as diffraction and other aberrations blur finer details. As opposed to a typical optical system, in Figure 4 we note that the retina-brain processing system labeled RTF enhances spatial frequencies from 2 to 6 cycles per degree. Receptive fields, on-off systems, and lateral inhibition are the well-known physiologic mechanisms that influence the different spatial frequency channels and are probably responsible for such enhancement.4–6 Why might middle to larger targets, that is, mid to low-spatial frequency targets, have an enhanced contrast sensitivity threshold? For example, the peak frequency of a normal subject's contrast sensitivity function is about 2 to 4 cycles per degree (cpd). From a survival standpoint some spatial frequencies might be more important than others. The quotation from the American Revolutionary War, “Don't fire 'til you see the whites of their eyes,” brings to mind a crucial facial feature, that is, the detection of eye movements. Given a palpebral width of about 24 mm and a central dark iris area of 12 mm in diameter, the white space from canthus to limbus is about 6 mm. Thus the eye itself can be likened to a thick dark bar straddled by two thinner white spaces framed by the darker skin. A person with a visual acuity of 20/200 would be able to see another person make a large eye movement at about 2 m away. Such clues might help to determine whether that person is friend or foe. Seeing facial expression at a few meters away also would be equivalent to seeing a target of about 3 cpd. Finally, a standing adult Figure ALMOST 100 yards away would also present a target equivalent to about 3 cpd. Being able to discern the presence of such a figure, particularly in rain or mild fog, could also have important survival value. Of course, the above examples merely suggest a possible reason for the unique shape of the contrast sensitivity function in which the spatial frequencies of 2 to 4 cpd are favored.
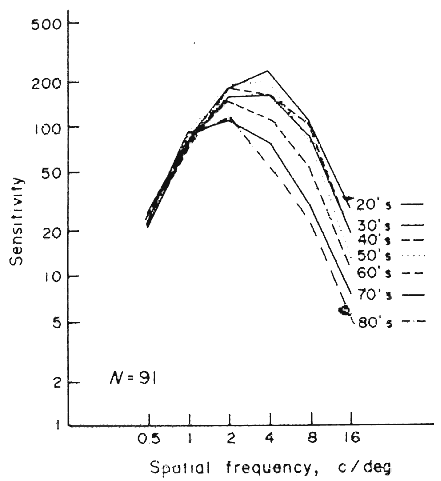
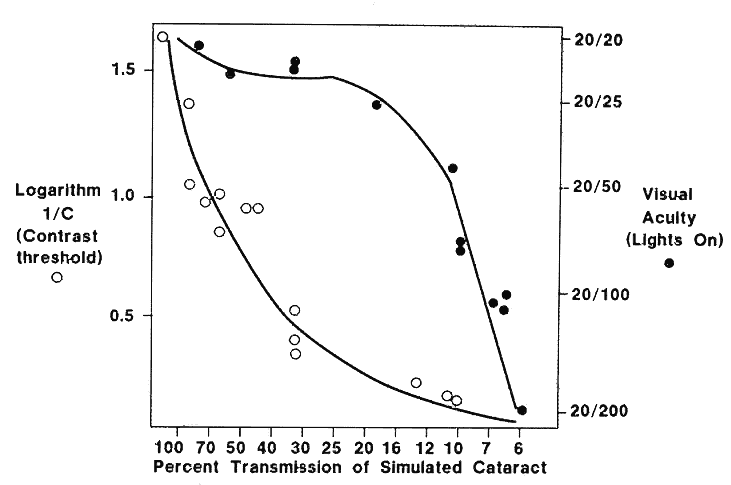
Normal variations are to be found in the human contrast sensitivity function. In Figure 5 contrast sensitivity is seen to decrease with age. Two factors appear to be responsible for the aging changes. First, the normal crystalline lens scatters more light with increasing age, thus blurring the edges of targets and degrading the contrast.7 Second, the retina-brain processing system itself loses its ability to enhance contrast with increasing age.8 The contrast sensitivity function also decreases substantially as the illumination in the scotopic range decreases.9 Finally, it should be noted that the contrast sensitivity impairment is a very sensitive indicator of cerebral or retinal disturbance due to high altitude hypoxia. Studies done on mountain climbers at elevations of over 17,000 feet in the Himalayan mountains demonstrate that a decrease of the contrast sensitivity was seen consistently in all subjects the day they arrived at base camp. The impairment was for the most part evident in the medial spatial frequencies. Contrast sensitivities normalized after 36 hours at the base camp.10
|
GLARE, TISSUE LIGHT SCATTERING, AND CONTRAST SENSITIVITY
In the body, collagen fibrils, interstitial fluids, and cellular elements all combine to make tendons white, dura opaque, and the epidermis translucent. Yet, combinations of the same ingredients are woven together in the eye to produce transparent corneas, lenses, and vitreous.11 Theoretically, these structures, particularly the corneas, should not be transparent. In the cornea clear, delicate collagen fibrils of index refraction 1.47 (close to that of glass) are surrounded by a mucopolysaccharide matrix with an index of refraction of about 1.33 (similar to water) according to research conducted by Maurice12 and Benedek.13 These two researchers found that the key to transparency is in the arrangement of the elements. Transparency can result from mixing two transparent elements of different refractive indices if the spacing between the elements is less than the distance of half a wavelength of light, and if there is a recognizable pattern or predictable relationship between the fibrils. A similar phenomenon can be seen in Figure 6 in which the diver has been made invisible by a blanket of air bubbles in the water. When a transparent structure loses its clarity, the physicist describes it as a light scatterer rather than a light transmitter. This concept is foreign to the clinician whose textbooks talk about opaque lenses and corneas. The word “opaqueness” conjures up the image of a cement wall that stops light. Of all the experiments demonstrating that most cataracts scatter light rather than stop light, the most graphic involves the relatively new science of holography. If it is true that a cataract splashes or scatters oncoming light so that a poor image is focused on the retina, then it should be theoretically possible to collect all the scattered light with a special optical element and recreate a sharp image. The essence of such an optical element, one that would take the scattered light of the cataract and rescatter it so that a proper image could be formed, would be a special inverse hologram of the cataract itself. Figure 7 shows how such a filter would work. Miller and colleagues14 were able to demonstrate how an extracted cataract (the patient's visual acuity was worse than 20/200) would be made relatively transparent by registering a special inverse hologram of that specific cataract in front of the cataract.
|
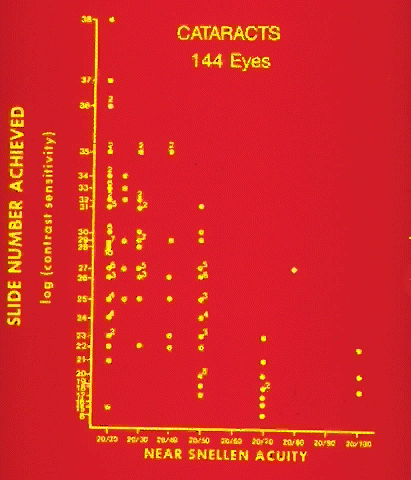
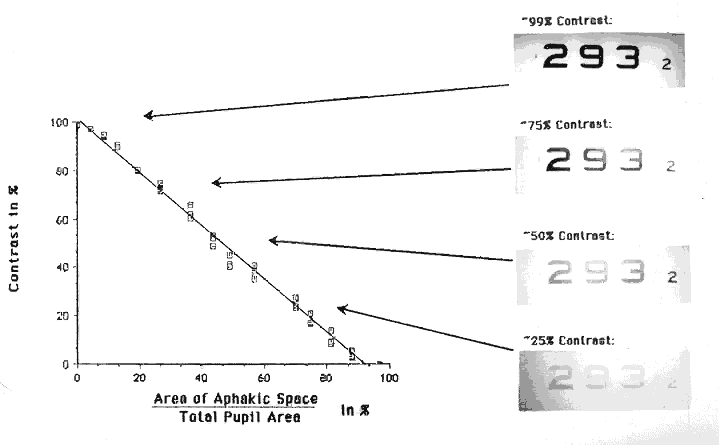
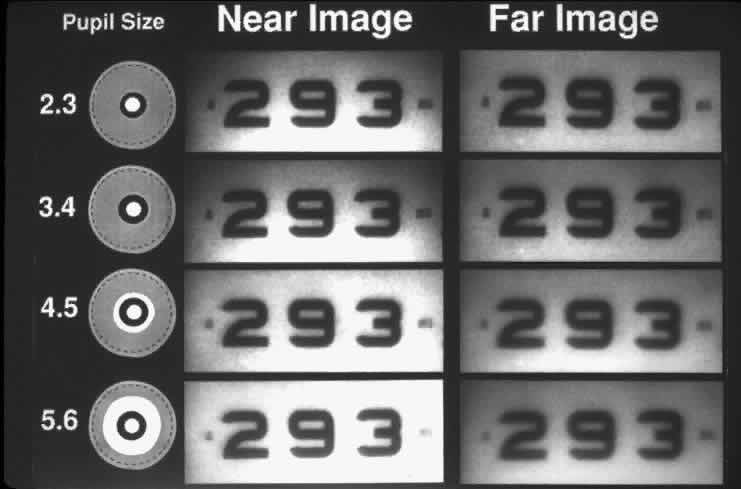
To follow the progress of conditions such as cataracts or corneal edema, a measure of tissue transparency or tissue backscattering is useful. Although it is possible to quantitate the amount of light scattered by various ocular tissues using photoelectric devices, a subjective discrimination system is needed to evaluate patient complaints. The Snellen visual acuity test was the traditional index, but it was not sensitive enough. In Figure 8, LeClaire and co-workers15 illustrated that many patients with cataracts showed good visual acuity but had poor contrast sensitivity in the face of a glare source. In fact, this should not come as a surprise, since the essence of vision is the discrimination of the light intensity of one object as opposed to another, often with a natural glare source present. Thus, a plane is seen against the sky because the retinal image of the plane does not stimulate the photoreceptors to the same degree that the image of the sky does. Terms like “contrast luminance” and “intensity discrimination” are used to describe differences in brightness between an object and its background.
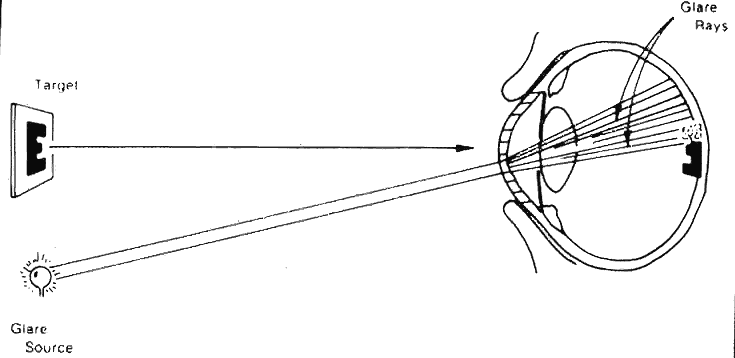
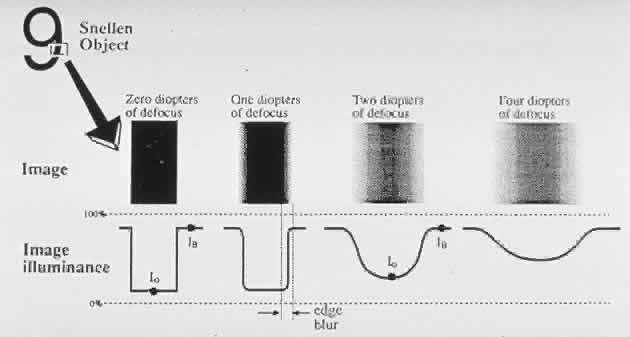
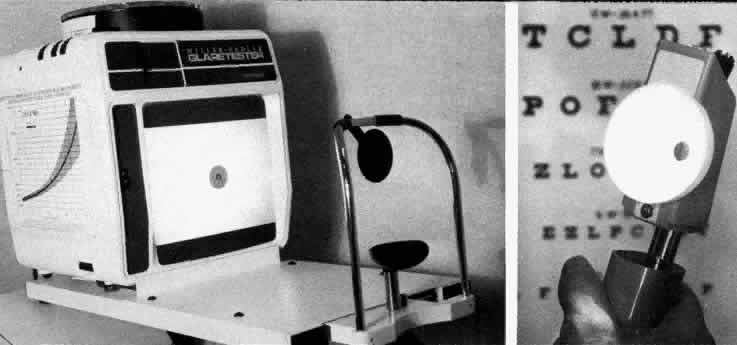
How then can ocular light scattering, glare and contrast sensitivity, be linked together to give the clinician a useful index? The stage had been set to solve this puzzle by an industrial scientist named Hollidy. In 1926, he developed the concept of glare and glare testing to measure the degrading effect of stray light.16 In the 1960s Wolfe, a visual physiologist working in Boston, realized that glare testing could be a useful way to describe the increase in light scattering seen in different clinical conditions.17,18 How does increased light scattering produce a decrease in the contrast of the retinal image in the presence of a glare source? Figure 9 shows how corneal edema splashes light from a naked light bulb onto the foveal image, reducing the contrast of the image of the target. Figure 10 illustrates the way that a patient with a cataract or corneal edema would see a scene in the presence of a glare source. In the mid-1970s, Nadler observed that many of his cataract patients complained of annoying glare. His observations rekindled interest in glare testing and led to the first clinical glare tester (the Miller-Nadler glare tester).