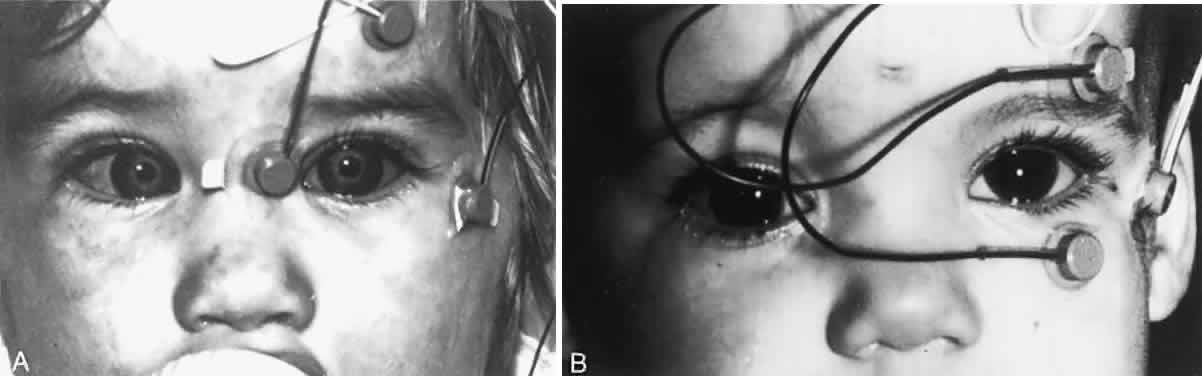
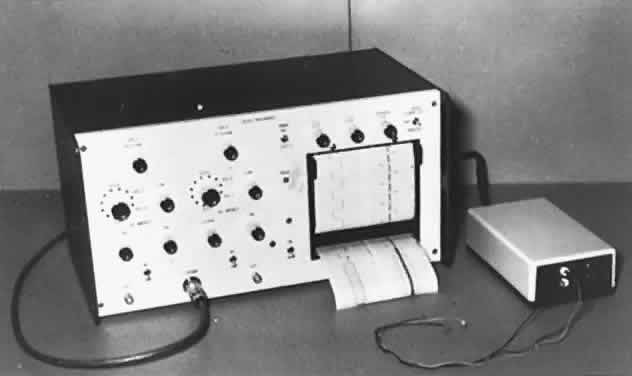
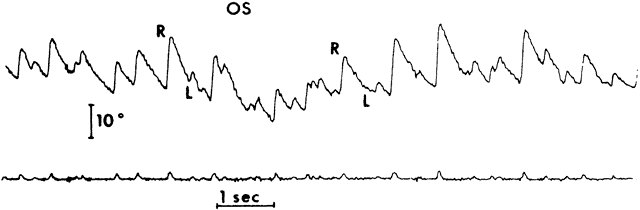
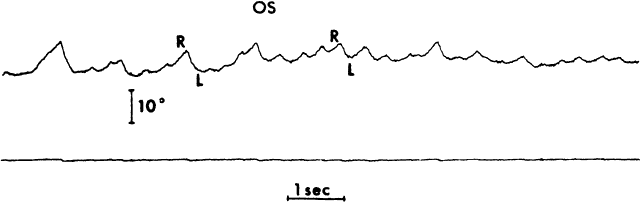
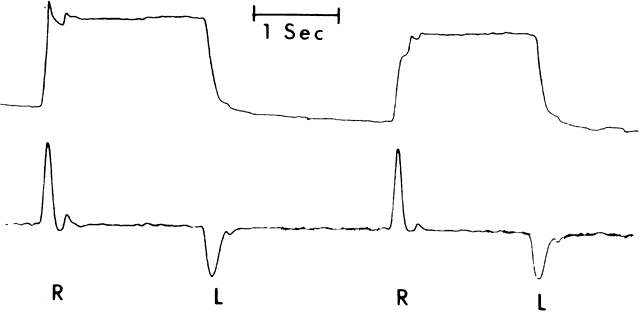
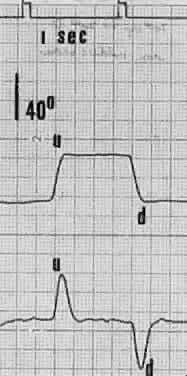
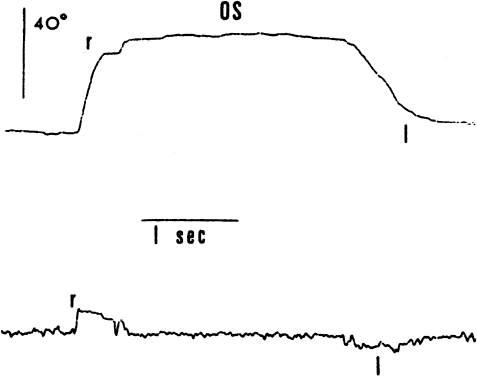
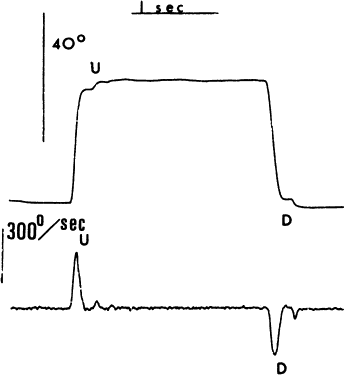
1. Dodge R: Five types of eye movements in the horizontal meridian plane of the field
of regard. Am J Physiol 8:307, 1903 2. Cook G, Stark L: Dynamics of the saccadic eye movement system. Commun Behavioral Biol 1:197, 1968 3. Tamler E, Marg E, Jampolsky A et al: Electromyography of human saccadic eye movements. Arch Ophthalmol 62:657, 1959 4. Young LR: Measuring eye movements. Am J Med Elec 2:300, 1963 5. Marmor MF, Zrenner E: Standard for clinical electro-oculography. Arch Ophthalmol 111:601, 1993 6. Metz HS: Calibration of saccades in infants. Invest Ophthalmol 25:1233, 1984 7. Metz HS: Saccadic velocity studies in paralytic strabismus: fixation with the paretic
vs the nonparetic eye. Ann Ophthalmol 17:37, 1985 8. Raab EL: Normal saccadic velocities. J Pediatr Ophthalmol Strabismus 22:20, 1985 9. Rosenberg ML: The indirect ophthalmoscope lens and eye movement disorders. Am J Ophthalmol 99:491, 1985 10. Metz HS, Scott AB, O'Meara D et al: Ocular saccades in lateral rectus palsy. Arch Ophthalmol 84:453, 1970 11. Rosenbaum AL, Kushner BJ, Kirschen DL: Vertical rectus muscle transposition and botulinum toxin to medial rectus
for abducens palsy. Arch Ophthalmol 107:820, 1989 12. Rosenbaum AL, Carlson MR, Gaffney R: Vertical saccadic velocity determination in superior oblique palsy. Arch Ophthalmol 95:821, 1977 13. Metz HS: Saccadic velocity studies in superior oblique palsy. Arch Ophthalmol 102:721, 1984 14. Boeder P: The cooperation of extraocular muscles. Am J Ophthalmol 51:469, 1961 15. Metz HS, Scott AB, Scott WE: Horizontal saccadic velocities in Duane's syndrome. Am J Ophthalmol 81:296, 1976 16. Hermann JS: Paretic thyroid myopathy. Ophthalmology 89:473, 1982 17. Feldon SE, Levin L, Liu SK: Graves' ophthalmopathy. Arch Ophthalmol 108:1568, 1990 18. Metz HS, Scott WE, Madson E et al: Saccadic velocity and active force studies in blowout fractures of the
orbit. Am J Ophthalmol 78:665, 1974 19. Metz HS: Double elevator palsy. Arch Ophthalmol 47:901, 1979 20. Scott AB: Strabismus: Muscle forces and innervations. In Lennestrand G, Bach
y Rita P (eds): Basic Mechanisms of Ocular Motility and Their Clinical
Implications, pp 181–191. Elmsford, NY: Pergamon Press, 1975 21. Dunlap EA: Vertical displacement of horizontal recti. In Dabezies (ed): Symposium
on Strabismus, pp 307–329. St. Louis: CV Mosby, 1971 22. Metz HS: Saccades with limited downgaze. Arch Ophthalmol 98:2204, 1980 23. Metz HS, Scott AB, O'Meara DM: Saccadic eye movements in myasthenia gravis. Arch Ophthalmol 88:9, 1972 24. Stella S: Optokinetic nystagmus in patients with ocular myasthenia. Invest Ophthalmol 6:668, 1967 25. Blomberg LH, Persson T: A new test for myasthenia gravis. Acta Neurol Scand 14(suppl 13):363, 1965 26. Spector RH, Daroff RB, Birkett JE: Edrophonium infrared optokinetic nystagmography in the diagnosis of myasthenia
gravis. Neurology 25:317, 1975 27. Yee RD et al: Rapid eye movements in myasthenia gravis. Arch Ophthalmol 94:1465, 1976 28. Koerner F, Schlote W: Chronic progressive external ophthalmoplegia. Arch Ophthalmol 88:155, 1972 29. Metz HS: Saccadic velocity measurements in internuclear ophthalmoplegia. Am J Ophthalmol 81:296, 1976 30. Dell'Osso LF, Robinson DA, Daroff RB: Optokinetic asymmetry in internuclear ophthalmoplegia. Arch Neurol 31:138, 1974 31. Siroky A, Krejcova H, Ymazal J: The early diagnosis of multiple sclerosis by monocular registration of
evoked nystagmus. Acta Neurol Scand 49:205, 1973 32. Abbott RL, Metz HS, Weber AA: Saccadic velocity studies in Möbius syndrome. Ann Ophthalmol 10:619, 1978 33. Van Allen MN, Blodi FC: Neurologic aspects of Möbius syndrome. Neurology 10:249, 1960 34. Lim L, Rosenbaum AL, Demer JL: Saccadic velocity analysis in patients with divergence paralysis. J Pediatr Ophthalmol Strabismus 32:76, 1995 35. Olmstead JMD et al: Adaptation to transposition of eye muscles. Am J Physiol 116:245, 1936 36. Metz HS, Scott AB: Innervational plasticity of the oculomotor system. Arch Ophthalmol 84:86, 1970 37. Metz HS: Saccadic velocity studies following rectus muscle transposition
surgery. In Kommerell O (ed): Proceedings of the Eye Movement Symposium
of the German Ophthalmological Society, pp 101–104. Munich: JF
Bergmann, 1978 38. Metz HS, Rice L: Human eye movements following horizontal rectus muscle disinsertion. Arch Ophthalmol 90:265, 1973 39. Rosenbaum AL, Metz HS: Diagnosis of lost or slipped muscles by saccadic velocity measurements. Am J Ophthalmol 77:215, 1974 40. Fletcher WA, Sharpe JA: Saccadic eye movement dysfunction in Alzheimer's disease. Ann Neurol 20:464, 1986 41. Nguyen N, Rimmer S, Katz B: Slowed saccades in acquired immunodeficiency syndrome. Am J Ophthalmol 107:356, 1989 42. Warabi T, Kase M, Kato T: Effect of aging on the accuraacy of visually guided saccadic eye movements. Ann Neurol 16:449, 1984 43. Robinson DA: Oculomotor unit behavior in the monkey. J Neurophysiol 33:393, 1970 44. Miller JE. Electromyographic pattern of saccadic eye movements. Am J Ophthalmol 46:183, 1958 45. Collins CC: The human oculomotor control system. In Lennestrand G, Bach
y Rita P (eds): Basic Mechanisms of Ocular Motility and Their Clinical
Implications, pp 145–180. Oxford, England: Pergamon Press, 1975 46. Mims JL, Treff G: Saccadic velocities of horizontal rectus muscles in twenty-five normal
humans. J Pediatr Ophthalmol Strabismus 19:129, 1982 47. Huber A: New techniques in diagnosis of eye muscle palsies: A review. J R Soc Med 73:115, 1980 48. Querre MA, Pecherean A, Lavenant F: Kinetic electro-oculography: Aims, disadvantages and limitations. Ophthalmologica 182:73, 1981 |