COVER TESTS
If a patient has a small deviation, detecting a small shift of the nonfixating eye to take-up fixation after covering the fixating eye is very suggestive of the monofixation syndrome. The shift would have to be 8Δ or less and is confirmed by the simultaneous prism and cover test. The alternate prism and cover test may reveal a larger deviation, but this does not negate the diagnosis of the monofixation syndrome as long as the simultaneous prism and cover test does not exceed 8Δ.
An absence of any detected movement by cover test is the finding in approximately one third of all monofixating patients. All possible responses may be found ranging from orthophoria, phoria, small tropia varying from 1 to 8Δ by cover-uncover and greater than 8Δ by alternate cover. The measurements by cover may vary in up- and down-gaze, in right- and left-gaze, and between distance and near fixation. The deviation may be eso, exo, vertical, DVD, or any combination, but a small eso shift is by far the most common cover test finding. However, the diagnosis of monofixation syndrome cannot be made solely with a cover test. Sensory tests also are required. The presence of a macular scotoma must be demonstrated in addition to the cover-test finding disclosing either no deviation or a deviation in ordinary seeing circumstances of no more than 8Δ. In order to demonstrate a macular scotoma in the nonfixating eye, it should be evident that the patient would have to have extramacular binocular vision, which is presumed to be NRC for reasons discussed in the preceding section.
WORTH 4 DOT TEST
A bifixating patient easily fuses the distant dots, but the scotoma in the monofixating patient obscures the dots projected into the nonfixating eye. Until the dots are projected onto a retinal area larger than the scotoma, the patient reports seeing either three green dots or two red dots. As the patient approaches the distant Worth dots, the retinal projection area of the images increases; and when it exceeds the size of the scotoma, suddenly the four dots are seen. The distance at which this occurs away from the dots allows an estimate of the size of the scotoma, since the projection angle of the dots is known for 6 meters. In patients capable of voluntarily fixating with either eye, the scotoma is illustrated in the visual field of either eye as they switch fixation from the green to the red dots and vice versa. The test is done just as well with a Near Worth 4 dot flashlight.
BINOCULAR PERIMETRY
Binocular perimetry is also used to study the scotoma. The binocular perimetric techniques may be build around a septum mirror or a projector apparatus that projects either color targets or Polaroid-treated targets on a screen that is viewed by the patients while wearing color filters or Polaroid analyzers. The projector technique is superior to the septum mirror technique because there is no target wand to distract the patient. In the author's system, a projector that shines a 1-mm sharply focused green light on a diffusely red illuminated screen is used. After placement of a red filter before one eye and a green filter before the other eye, the patient fixates a 5-mm black “O” target at 1 meter in the center of the screen. The screen has a 5° concentric black circle surrounding the central target, and the patient is directed to centrally position the green target within the fixation target. The scotoma is manifest by the disappearance of the green test target as it approaches the fixation target. The position, shape, and size of the scotoma are determined by bringing the test target in along various isopters toward the fixation target and reporting when the test target disappears. Children move the mounted movable green projector as if it were a mounted gun. They find this binocular perimetric technique entertaining, and they quickly plot their own scotoma. Binocular scotometry is done in a room darkened except for illumination from the red light and green light projector. Since the red-green filters dissociate the eyes in a darkened room, binocular control of the alignment is lacking during this test. Hence, the scotoma is positioned in reference to the fixation target according to the deviation of the eyes disclosed by the alternate cover test. In orthophoric patients, the scotoma is centered around the fixation target; in patients with esodeviation, it is displaced heteronymously; and in patients with exodeviation, it is displaced homonymously. With this test, NRC is invariably demonstrated in patients with the monofixation syndrome.
Patients with bifixation have a dramatically different response to binocular perimetry than patients with monofixation. Those with bifixation superimpose the green test target on the fixation target without hesitation. However, unless the patient is orthophoric, the test target is displaced from the fixation target according to the point at which the visual axis of the nonfixating eye strikes the screen when superimposition of the targets is claimed. In contrast, patients with monofixation manifest frustration as the test target disappears during its approach toward the fixation target. These patients usually make many approach attempts before conceding that the test target consistently disappears at a point approximately 1.5° to 2.5° short of the fixation target.
The scotoma can be plotted by the green projector and red filter technique in almost all patients having the monofixation syndrome. The scotoma is probably always in the visual field of the nonfixating eye, but some patients find it impossible to hold fixation of the nonpreferred eye on the fixation target as the test target approaches it. As the target reaches the boundary of the scotoma, some patients surrender to the compulsion to switch fixation from the fixation target to the test target. Instead of the test target being within the scotoma, the fixation target is located there, and any opportunity to plot the scotoma in the preferred eye is lost. Amblyopia is a definite factor that interferes in maintaining fixation of the fixation target with the nonpreferred eye; the severity of the amblyopia is directly proportional to the degree of difficulty in maintaining fixation on the fixation target.
4Δ BASE-OUT PRISM TEST
The 4Δ base-out prism test described by Irvine10 is another method frequently used to reveal the scotoma in patients with the monofixation syndrome. While the patient reads letters at a distance of 6 meters, a 4Δ base-out prism is slipped before first one eye and then the other. The prism-covered eye is watched closely for movement. Absence of movement by one of the eyes is proof of a macular scotoma in that eye. Bifixation is identified by each eye moving inward to fixate in response to the image displacement produced by the prism. The test is not completely reliable because occasionally bifixating patients recognize diplopia when the prism is slipped before either eye, but make no attempt to restore bifixation by convergence. The patient who manifests a shift to cover-uncover usually gives a positive response for a scotoma with the 4Δ base-out prism test, while a large percentage of the patients having no shift to cover-uncover respond negatively to this test. Possibly the explanation for this fact is that the monofixating patient with no shift to cover-uncover is more apt to switch fixation from one eye to the other when the 4Δ base-out prism is placed before the fixating eye than to refixate this eye after fixation is broken by the sudden prismatic shift of the visual field. Patients without amblyopia and without a shift to cover-uncover are particularly prone to yielding a negative 4Δ base-out finding. When the test works it is excellent, but there is always a large percentage of the monofixating patients who respond equivocally or negatively to this test.
BAGOLINI STRIATED GLASSES TEST
The Bagolini striated glasses test is another technique for disclosing the invariable scotoma in the visual field of the nonfixating eye in patients with the monofixation syndrome. Patients are taught to recognize their own scotoma and report on it while viewing a small hand-held muscle light 15 inches away in a normally illuminated room. The striations on the glass produce a sharp bright streak of light emanating from the light source across the entire visual field perpendicular to the glass striations. The glasses are positioned before each eye so that the streaks are perpendicular to one another in the binocular visual field. Oblique placement of the streaks is best, since this allows part of each streak to be on both the nasal and the temporal retina.
The glasses are positioned so that the streak seen by the right eye is at 135° and the streak seen by the left eye is at 45°. The transparency of the striated glasses offers two advantages over other testing techniques: first, the glasses allow a normal environmental test situation, and second, the examiner can evaluate simultaneously the ocular alignment and the patient's sensorial response. Most patients with the monofixation syndrome (if they are observant) see a scotoma as a gap around the light in the streak seen by the nonfixating eye. A little more of the streak on one side or the other of the light may be missing, and this is somewhat related to the deviation. Often more of the streak projected onto the nasal retina is missing in patients with esodeviation, and more of the streak projected onto the temporal retina is missing in those with exodeviation. The gap around the fixation light, projected onto a grid, indicates a scotoma of 3° to 5°. Until the patient's attention is directed to it, the break, or gap, is visually overlooked. It remains unrecognized in a manner similar to physiologic diplopia until the patient is made aware of it. In studying the scotoma in the visual field of one eye with the Bagolini striated glasses technique, the patient is encouraged to switch fixation to the other eye to observe whether or not the scotoma has been transferred to the visual field of the other eye.
A-O VECTOGRAPHIC PROJECT-O-CHART SLIDE
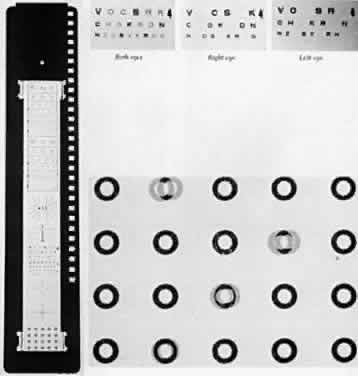
The A-O Vectographic Project-O-Chart Slide* (Fig. 1) is another method for the study of a scotoma in patients with the monofixation syndrome. It is used in conjunction with a nondepolarizing aluminized screen. The polarized letters of the Polaroid Vectograph slide provide a rapid and dependable differentiation between patients with bifixation and those with monofixation. Each character on the slide has self-contained light polarizations; some are polarized at 90° to others. Viewed through analyzers, some images are made visible to one eye and invisible to the other, while some characters are visible to both eyes. This method provides a test environment closely approximating the normal binocular situation. The patient with bifixation reads the entire 20/50 (6/ 15)† visual line without hesitation, although two letters are seen only by the right eye, two others only by the left eye, and the remaining two letters by both eyes. The patient with monofixation deletes the two letters that are imaged only in the nonfixating eye. Occasionally, the monofixating patient who rapidly alternates fixation from one eye to the other reads all six letters, but usually comments that as two letters disappear two others appear. This response misleads the examiner if the patient does not spontaneously comment about the everchanging letters appearing and disappearing.
* American Optical Company.
† Metric equivalent in parentheses after Snellen notation.
STEREOACUITY TESTS
Stereoacuity is measured in seconds of arc of image disparity. The factor determining the stereoacuity in the patient with monofixation syndrome is the macular scotoma in the visual field of the nonfixating eye. Bifixation allows the high resolving powers of each macula to detect minute degrees of retinal image disparities, yielding a stereoacuity range between 14 and 40 seconds of arc. In monofixation, the retinal image disparity is detected by studying the images on retinal areas having low resolving power, giving stereoacuities between 60 and 3000 seconds of arc. It has been stressed that stereoacuity is a reliable indicator of either monofixation or bifixation.11
Polaroid vectographs offer a convenient, accurate, and simple method to determine stereoacuity. The vectographs produce the image disparity, and the patient sees them through polaroid analyzers in normal room illumination. If the patient wears glasses, the analyzers are fitted over them. The stereoacuity is measurable at either 6 m or 40 cm.
Distant stereoacuity is measured by the A-O Vectographic Project-O-Chart Slide (see Fig. 1) that presents a range of stereotargets between 240 and 60 seconds of arc.
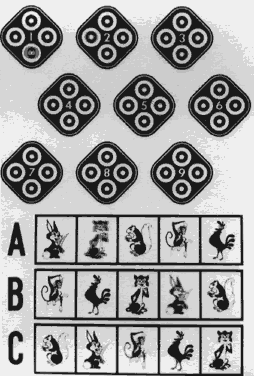
Near stereoacuity is measured with Polaroid vectographs by either the Titmus Stereotest* or the Randot Stereotest†. The house fly Titmus Stereotest (Fig. 2) produces approximately 3000 seconds of arc of retinal image disparity at 40 cm. The circles of the Stereotest (Fig. 3) presents a range of stereotargets producing retinal image disparity between 800 and 40 seconds of arc at 40 cm.
* Titmus Stereotest, Stereo Optical Company, Inc, Chicago, IL
† Randot Stereotest, Stereo Optical Company, Inc, Chicago, IL
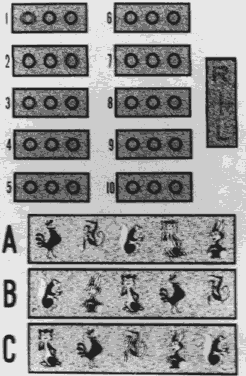
The circles in the Randot Stereotest present random dot stereotargets (Fig. 4) as opposed to the contoured targets of the Titmus Stereotest circles. The Randot targets provide a range of retinal image disparity between 400 and 20 seconds of arc at 40 cm. The Randot eliminates the lateralization clues of Titmus circles that can give false—positive results. Another random dot near stereoacuity test is the TNO Stereotest ‡ utilizing color targets and analyzers rather than Polaroid vectographs to produce the retinal image disparity, giving a range between 480 to 15 seconds of arc.
‡ TNO Stereotest, Lameris, Utrecht, The Netherlands