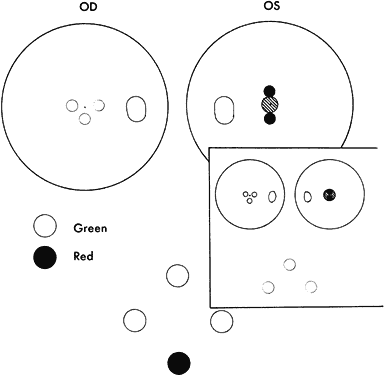
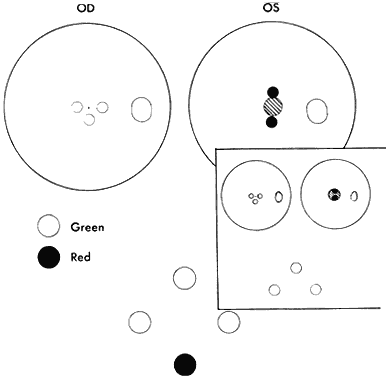
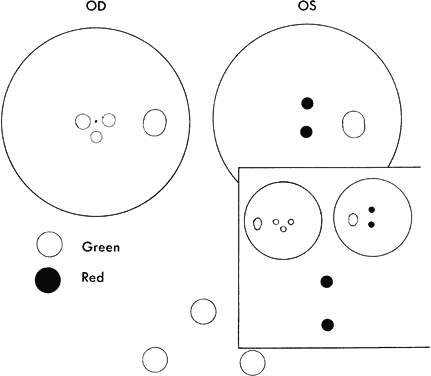
WORTH 4-DOT TEST
One of the simpler methods for investigating fusion, suppression, and anomalous retinal correspondence (ARC) is the Worth 4-dot test. The testing target consists of four illuminated dots that are clustered equidistant from each other; two dots are green, one is red, and one is white. The patient views the target through anaglyphic testing glasses that consist of a red filter in front of one eye and a green filter in front of the other. Viewed through the red filter, the green dots are invisible; viewed through the green filter, the red dot is invisible. The white dot is seen as red when viewed through the red filter and as green when viewed through the green filter. The test subject monocularly sees two red dots through the red filter and three green dots through the green filter. Binocularly, however, the fusing subject perceives the target as four dots because the white dot is seen as either a single red or green dot, according to which eye is dominant. If the dominance pattern vacillates between the eyes, the white dot manifests color rivalry by changing from red to green.
The examiner must consider that the response of the patient may vary according to the size of the projection angle that the cluster of the four dots presents to the retinas. A normal subject possessing both macular and extramacular binocular vision perceives four dots, regardless how minute the projection angle that the target subtends on the retina. A patient devoid of macular binocular vision but who possesses extramacular normal binocular vision does not manifest fusion of the target (subtending an angle of about 3° or less) yet manifests a fusion response for larger targets.
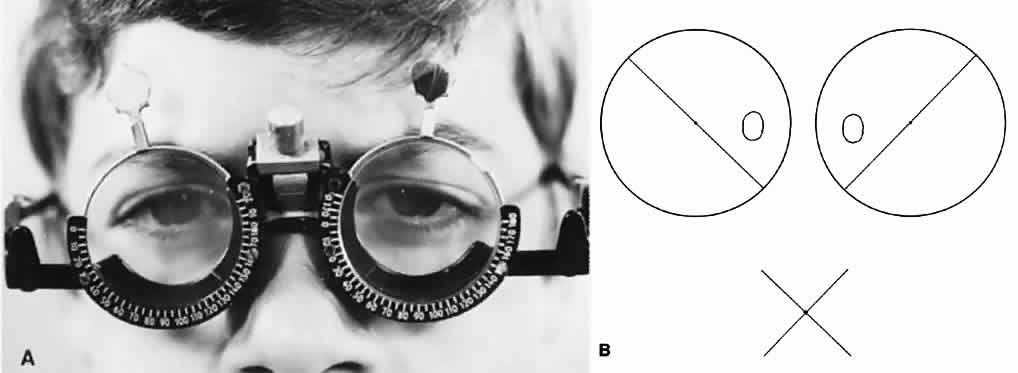
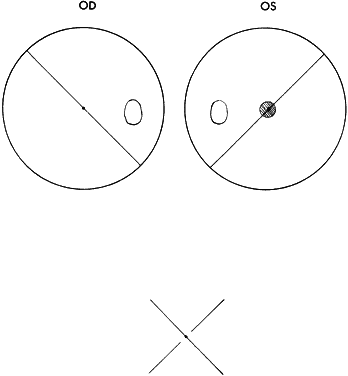
Essentially, two methods of performing the test are available. One is to have a fixed target at a distance from the subject, which may either be contained in an illuminated box or projected on a screen. This is referred to as the distant Worth dot test. The other method is a near Worth dot test, which consists of a flashlight easily advanced or receded from the subject to alter the projection angle of the target image on the retinas. The latter can be accomplished with the fixed location of the distant Worth dot test by having the subject advance toward or recede from the target. At 6 meters, the distant Worth dots project an image of 1.25°; at 0.33 meters, the near Worth dots project a 6° angle (Fig. 1). The projection angle of the image is the imaginary circumference encircling the outer border of the four dots collectively, not simply the projection angle of each of the four dots. The subject should be tested with the optimal optical correction on (spectacles or contact lenses) behind the anaglyphic filters.
|
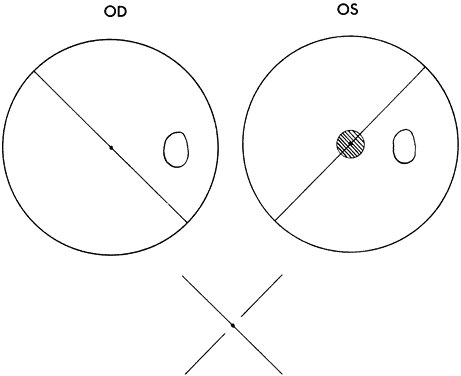
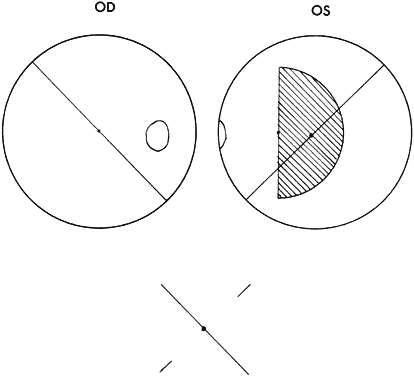
Monofixation syndrome patients have a 3° macular scotoma in the nonfixating eye (Fig. 2), which precludes a fusion response unless the projection size of the target image exceeds 3°. If the distant Worth dot test is 6 meters from the patient, it is not fused. By advancing toward the target, fusion is achieved at about 2.5 meters. Because the standard near Worth-dot flashlight projects 6° images at 0.33 meters, the test should begin about 2 meters from the patient. On advancing the target toward the patient, fusion is usually manifest at about 0.66 meters. Fusion response is lost by moving the flashlight away from the patient farther than 0.66 meters, and the patient sees either only two red or three green dots, according to which is the fixating eye. When this occurs, the examiner is inclined to state or to record this response as “suppression,” which it is not. The test result merely identifies the presence of the macular scotoma in the nonfixating eye of the monofixation syndrome patient. Whatever the cause of the macular scotoma in the monofixation syndrome patient—dissimilarity of macular images exceeding the threshold tolerated for macular fusion, an inherent inability to develop macular binocular vision (bifixation) despite having developed extramacular binocular vision, or a unilateral macular destructive disorder—it is not a scotoma generated by a cortical process to eliminate symptoms of diplopia and visual confusion, as encountered in strabismus. The latter is suppression. Its scotoma is displaced away from the macula in the nonfixating eye's extramacular retina locus that receives the image projected onto the fixating eye's macula. The suppression scotoma in the strabismic patient is larger than the macular scotoma in the monofixation syndrome patient. The suppression scotoma in the strabismic patient is associated with ARC, whereas the macular scotoma in the monofixation syndrome patient is associated with normal retinal correspondence (NRC). The strabismic patient manifesting a suppression scotoma does not have stereopsis. The suppression scotoma exists in the strabismic patient because the extramacular binocular vision reflex has the ability to adapt to any strabismic angle and continue to fuse by changing from its innate NRC to ARC plus, developing suppression. The macular binocular vision reflex is devoid of this attribute. The development of any deviation of the fixation axes of the eyes of more than 20 minutes of arc instantly causes the reflex to cease functioning, manifesting no annoying symptoms of diplopia and visual confusion but having no capacity to adapt to the strabismic deviation of the eyes and continue functioning.
|
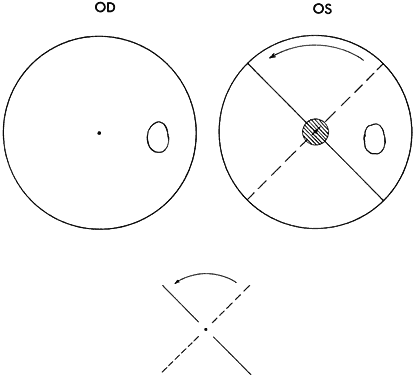
Strabismic patients who acquire deviation of 10Δ or more after having developed normal binocular vision reflexes have diplopic and visual confusion symptoms until extramacular binocular vision reflex converts from NRC to ARC. While still in NRC mode, the response to the Worth dot test is five lights (two red and three green). The dots seen by the fixating eye are clear, whereas those seen by the deviating eye are blurred. The blurred dots are projected in space opposite the direction of the deviated eye, causing homonymous diplopia in esotropia, heteronymous diplopia in exotropia. The dots seen by the hypertropic eye appear lower; those seen by the hypotropic eye appear higher. The image in the intorted eye appears extorted; that in the extorted eye appears intorted. Horizontal or vertical diplopia can be eliminated by the appropriate prism or prisms placed before the strabismic eyes but there is no prism correction that eliminates the torsional diplopia. As the ARC fusion mode gradually replaces the NRC fusion mode, the Worth-dot response becomes four lights—despite strabismic deviation. The projection angle on the cluster of dots, however, must exceed the size of the suppression scotoma that always accompanies ARC. The suppression scotoma is 5° or more in esotropia (Fig. 3) but larger in exotropia.
|
The esotrope's suppression scotoma is usually overcome by advancing the near Worth-dot flashlight to about 40 cm from the eyes, with the patient at that time giving a fusion response. As the flashlight recedes beyond 40 cm, the cluster of dots project within the boundary of the suppression scotoma, and only those dots projecting into the fixating eye are seen. In exotropia, the suppression scotoma is larger and extends up to the hemiretinal line. Consequently, it is unusual to succeed in projecting the near Worth-dot flashlight cluster outside the large temporal retina scotoma. Hence, the test generally does not validate ARC in the exotropic patient because the fusion response is seldom obtainable. For this reason, the sensorial adaptations in the exotropic patient are better investigated by methods other than the Worth 4-dot test alone. For example, ARC in the exotropic patient during either near or distant Worth-dot testing may be revealed as the base-in prism power neutralizing the objective angle that is placed before the eyes. As soon as the cluster of dots is moved across the hemiretinal line and out of the temporal scotoma, homonymous diplopia is elicited despite the targets being projected on corresponding retinal areas. The patient sees the diplopic images as being separated by a considerable distance.
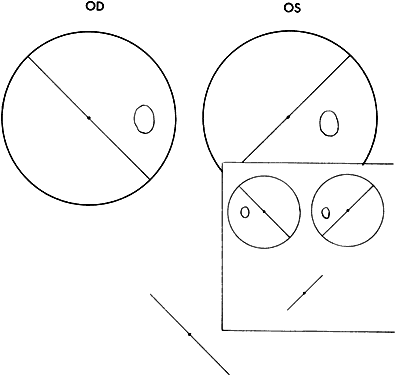
Absence of binocular vision is manifest in the esotropic patient when regardless how near the target to the eyes or how large the projection angle of the cluster of dots, the patient continues to see either three green or two red dots (Fig. 4). One precaution must be exercised by the examiner to guard against rapid alternation of the fixating eyes, which can produce a response of five dots because of summation of the three green dots seen by one eye and the two red dots seen by the other eye. The important question is whether the five lights are seen simultaneously or whether the patient is adding the separate responses?
The Worth 4-dot test is simple enough to be performed on children who can count to five. If the visual acuity can be determined, so can the Worth 4-dot response. The test is performed with ordinary room illumination to provide the usual peripheral vision clues.
BAGOLINI STRIATED GLASSES TEST
Most tests for fusion, suppression, and ARC create artificial viewing circumstances. Normally, the visual environment is not that of a red filter in front of one eye or a combination of red-green filters; separately viewed slides in illuminated tubes are nothing more than a laboratory analysis of retinal correspondence. The striated glasses popularized by Bagolini, however, allow the patient to view the normal visual environment with a faint reference line placed on the background viewed by each eye (Fig. 5). The reference line for each eye is placed at right angles by arranging the glasses in the trial frame so that the striations before the right eye and the left eye are perpendicular to each other. For example, the striations are placed at 135° in the trial frame in front of the right eye and at 45° in front of the left eye. The patient views a fixation light at any distance the examiner chooses; ordinary room illumination is maintained. The patient reports on the fixation light and observed streaks extending out into the peripheral field of vision.
|
Patients with either straight eyes or with a deviation up to 8Δ by simultaneous prism and cover test and NRC binocular vision describe seeing one fixation light and two streaks forming an X intersecting at the light. The bifixating patient with macular fusion sees it as just described. If macular fusion is lacking although extramacular fusion is present, a gap of 3° is reported in one streak on either side of the light (Fig. 6). The gap represents the macular scotoma in the nonfixating eye of the monofixating patient. Monofixating patients are unaware of this gap in the one streak around the fixating light until the examiner questions them about it; they invariably seem to be oblivious to the presence of the scotoma until it is brought to their attention. When the fixating eye is covered, the gap in the streak disappears, and the entire streak then passes through the center of the fixation light. If the patient can switch fixation, the macular scotoma is observed to transfer from eye to eye.
|
Patients with esotropia of 10Δ or more give varied responses, depending on whether they have NRC or ARC monocular vision or an absence of binocular vision. The NRC esotropic patient sees two fixation lights in homonymous diplopia, with a separate streak through each and without a break in either streak. Compensating for the esotropic angle with base-out prisms eliminates the diplopic fixation light, and the streaks then intersect at the fixation light. The patient with ARC and suppression sees one fixation light and two streaks forming an X; after questioning, the patient recognizes the suppression scotoma projecting from the nasal retina of the deviated eye as a gap of 5° to 6° around the fixation light in the streak seen by that eye (Fig. 7). The scotoma can be further studied by removing the striated glass from in front of the fixating eye and slowly rotating the striated glass before the nonfixating eye (Fig. 8). As the streak rotates, the gap in the streak around the fixation light persists, beautifully outlining the scotoma for 360°. Furthermore, ARC is made evident by the patient's claim that the streak seen by the deviated eye passes through the fixation light as the patient mentally connects the two ends of the gap in this streak. When the light is held in front of the eyes, base-out prism power equal to the esotropic deviation produces crossed diplopia for the fixation light, and each light has its separate streak passing through it. The patient devoid of single binocular vision sees only one light and one streak (Fig. 9). The patient may claim to see two streaks if rapidly alternating but will admit under questioning that they are not perceived simultaneously.
|
|
|
The patient with exotropia of 10Δ or more may report NRC with heteronymous diplopia, ARC with suppression, or an absence of binocular vision. The large profound scotoma of the temporal retina, extending up to the hemiretinal line in the exotropic patient with ARC, prevents all but the best observers from appreciating the extremely peripheral small streak seen outside the suppression scotoma of the deviated eye (Fig. 10); consequently, many exotropic patients report seeing only one streak. Those who can detect the small peripheral ends of the streak describe the ends on the axis that coincides with the light, supporting the diagnosis of ARC. Furthermore, base-in prism power placed in front of the eyes that equals the deviation angle creates homonymous diplopia of the fixation light, each image having a separate streak.
|
The Bagolini striated lens test requires a degree of maturity that is seldom found in a child younger than 8 years of age. Describing or drawing the suppression scotoma gap in one of the streaks presents great difficulty to the young child.
MAJOR AMBLYOSCOPE TEST
Fusion is investigated with the major amblyoscope by placing appropriate fusion targets in the housing of each tube and adjusting the instrument to project the similar images onto corresponding retinal areas of each eye. The fusion targets are identical except for one detail. For example, the target may be a clown that is identical in every detail on the right and left slides except that the hat is lacking from the right slide and the cane from the left. The report that the clown has both the hat and the cane indicates fusion. It is a great advantage that the tubes of this instrument can be adjusted according to the strabismus angle, enabling the targets to project onto corresponding retinal areas.
After the presence of sensory fusion has been ascertained with this instrument, further testing is natural to investigate the motor aspect of fusion by determining the fusional vergence amplitudes. The technique for this determination is described elsewhere in these volumes.
Retinal correspondence in a strabismic patient is also investigated on the major amblyoscope. For this test, the targets must be dissimilar—for example, a fish and a bowl. If the patient has binocular vision, the dissimilar images projected on each retina are simultaneously perceived. The tubes are set at the objective angle (the adjustment that compensates for all refixation movements of the eyes as the tubes are alternately illuminated while the center of each target is alternately fixated), which measures the angle of the strabismus. Next, the patient is asked to adjust the tubes so that the targets appear superimposed; this reveals the subjective angle. Objective and subjective angles that are identical indicate NRC; those that are dissimilar indicate ARC. A subjective setting of zero by a strabismic patient is harmonious ARC, indicating that the cortical adaptation of ARC fully compensates for the angle of strabismus. A subjective angle that is somewhere between zero and the objective angle is unharmonious ARC, indicating that the cortical adaptation of ARC does not fully compensate for the angle of strabismus. Actually, the ARC patient usually experiences some difficulty in superimposing the targets at the subjective angle (e.g., as the patient approaches the lion toward one end of the cage it suddenly disappears, only to reappear as it leaves the other end of the cage) because of the suppression scotoma coexisting with ARC.
The advantages and disadvantages of the major amblyoscope test should be described. The advantages are (1) the tubes can be adjusted to compensate for the combined horizontal, vertical, and torsional deviations of the eyes; (2) the fusional vergence amplitudes are readily measurable for each of the three planes; and (3) enhancement of their amplitudes may be treated on this instrument. The disadvantages are that the instrument induces convergence despite the 5-diopter lens in the viewer's end of the tubes and that the distance between lens and target in the housing is 20 cm. Awareness by the patient that the target is near induces accommodation, which is associated with accommodative convergence. The result is that the convergence induces either greater esodeviation or less exodeviation than the prism and alternate-cover measurement that were obtained while the patient viewed an actual distant target. Second, although stereopsis or its absence is ascertainable on this instrument, measuring the stereoacuity accurately is nearly impossible. Third, the concepts regarding binocular vision that have evolved around this instrument are inaccurate and pervasive. The three grades of fusion described by Worth1 (i.e., simultaneous perception, fusion, and stereopsis) create a barrier for understanding binocular vision because neither simultaneous perception nor stereopsis are fusion. Also, the finding of ARC not matching the objective angle of the strabismus deviation, creating the classification of harmonious and unharmonious ARC, can be proved inaccurate by the Bagolini striated glasses test. The essential difference is that viewing through Bagolini striated glasses discloses only harmonious ARC. The laboratory test of viewing through tubes—without peripheral vision clues—rather than viewing in real space confuses the patient, leading to the artifact of unharmonious ARC.
RED FILTER TEST
Fusion is tested by viewing a muscle light with both eyes while a red filter is maintained in front of one eye. A nondiplopic response and a report of a pink light suggest macular fusion. Individually, one eye sees the light as white, whereas the other sees it as red, but in accordance with the laws of color fusion, the fused perception is a pink tint.
Scotoma is tested for by placing the red filter in front of the nonfixating eye. A report that only the white light is seen suggests that the red image is projecting within the scotoma. The alignment of the eyes determines the retinal locus from which the scotoma projects. In patients with straight eyes or in those whose deviation by simultaneous prism and cover is 8Δ or less, the scotoma includes the macula; in strabismic patients with greater deviations, the scotoma projects from an extramacular region.
Size and profoundness of the scotoma are investigated next. The nasal scotoma in esotropia is about 5° to 6°, whereas the temporal scotoma in exotropia is usually larger and extends up to the hemiretinal line. The depth of the scotoma may vary from slight to profound. Rapidly covering and uncovering the eye with minimal suppression for just a few moments may be sufficient to produce appreciation of diplopia. If suppression persists despite flashing of the occluder, the examiner introduces a 15Δ base-up or base-down prism in front of the red filter. This is usually sufficient (in esotropic patients at least) to move the red image out of the suppression area. If diplopia still is not reported, the examiner wiggles the prism in a rocking manner; this constantly moves the image on the retina. If many attempts with the latter technique are required to awaken diplopia, the suppression scotoma is indeed profound. In many cases of esotropia, the scotoma is so profound that diplopia is never recognized by the patient.
Once diplopia can be maintained, the examiner can proceed with an analysis of the retinal correspondence, even if a 15Δ base-down prism must be held constantly before the eye behind the red filter. First, the horizontal nature of the diplopia must be established. The absence of any horizontal displacement when there is a horizontal strabismus, regardless of whether it is esotropia or exotropia, is diagnostic of harmonious ARC. Horizontal displacement of the lights, which is homonymous for esotropia and heteronymous for exotropia, indicates there is either NRC or unharmonious ARC. To differentiate, base-out prism power for esotropia and base-in power for exotropia is introduced until the white and red lights are seen without horizontal separation. A convenient method for introducing this prism power is to use either the horizontal prism bar or the rotary prism. If the strabismus angle and the prism power required to align the diplopic images vertically are about the same, the patient has NRC; otherwise, the patient has unharmonious ARC. The test is more likely to produce an unharmonious ARC result if conducted in the dark without real-space peripheral vision clues. Some confusion will always occur, however, because patients are unaccustomed to viewing with one eye, to contending with a different color environment, and to reduced ambient light.
LANCASTER PROJECTORS
Another method of investigating the retinal correspondence in a strabismic patient entails the use of the Lancaster red-green projectors. The red-green filters used in the Worth 4-dot test are worn by the patient. One projector shines a red target and the other a green target on a calibrated screen 1 meter from the patient. Controlling the adjustment of the projectors while keeping one on zero (screen center), the patient arranges the targets so they become superimposed (subjective angle). The calibrated screen offers the examiner a direct reading of the subjective angle of the patient. If the examiner already knows the objective angle from prism and alternate-cover testing, a comparison is readily made between the objective and the subjective angle. The two angles are equal in NRC and dissimilar in ARC. Superimposition of both the red and the green targets on zero indicates that ARC is harmonious. Superimposition of the targets at an angle between the angle of strabismus and zero indicates unharmonious ARC.
AFTERIMAGE TEST
The retinal correspondence in older strabismic children who have central fixation ability in each eye may be investigated with the afterimage test. A linear electric filament about 30 cm long is the basic component of the instrument. An area about 4 cm long in the center of the filament is blocked out, so that when the filament is turned on, it appears as two illuminated filaments 13 cm long, separated by a 4-cm nonilluminated gap. The patient's attention is directed to a fixation spot in the center of the gap. The illuminated filament is offered vertically for about 15 seconds to the right eye while the left eye is shielded and then horizontally for the same time to the left eye while the right is covered (Fig. 11). This technique produces vertical and horizontal afterimages, each having a central gap. Positive afterimages are seen with either eye closed or in the dark, whereas negative afterimages are experienced with the eyes open in an illuminated room. Negative afterimages are best brought out by intermittent illumination (i.e., by flickering the lights off and on) because these images tend to wash out rapidly in sustained illumination. A report from the patient that the afterimages form a cross signifies NRC. A vertical afterimage line that is displaced away from the break in the horizontal afterimage line is found in ARC. In esotropia with ARC, the afterimages are heteronymous; the image seen by the right eye is left and that by the left eye is right. The images are homonymous in exotropia with ARC. Occasionally, the patient with tropia reports NRC with positive afterimages and ARC with negative afterimages. This peculiar finding means that the patient uses the innate NRC system when the eyes are not exposed to clues in the environment but shifts to the adapted ARC system when the eyes are in the open. The implication of this finding is that the prognosis is better for a return to fusion with NRC after the squint angle has been eliminated than the prognosis in the case in which ARC is present with both positive and negative afterimages.
|