PARAMEDIAN PONTINE RETICULAR FORMATION
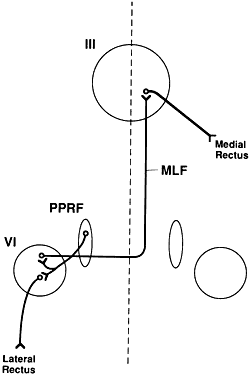
The paramedian pontine reticular formation (PPRF)is the primary center responsible for generatinghorizontal conjugate gaze. The PPRF is positionedventral to the medial longitudinal fasciculus (MLF). It extends from the level of the trochlear nerve nucleus to the abducens nerve nucleus. Its major efferent projections are to the ipsilateral abducens nucleus (Fig. 1). Secondary efferent projections are to the rostral interstitial nucleus of the MLF (riMLF), which controls vertical gaze. Most afferent connections to the PPRF are from the vestibular nuclei, but there also is input from the cerebellum, superior colliculus, and frontal eye fields (FEF).
Three types of cells have been identified in the PPRF: excitatory burst cells, inhibitory burst cells, and pause cells. The excitatory burst cells generate ipsilateral horizontal saccades by way of projections to the ipsilateral abducens nucleus. The axons from these cells synapse in the abducens nucleus onmotor neurons that innervate the ipsilateral lateral rectus and on interneurons that innervate the contralateral medial rectus subnucleus by way of the contralateral MLF (see Fig. 1). Burst cells discharge only when there is need for a fast eye movement and do not discharge during fixation, pursuit, or vergence eye movements.
Inhibitory burst cell axons have their synapse in the contralateral abducens nucleus. Stimulation of these neurons decreases the firing rate of those motor neurons and interneurons, thereby inhibiting the antagonist muscles of the intended eye movement. Their firing rate is inversely proportional to the burst cells.
Pause cells tonically discharge except when a saccade is being generated. These cells inhibit the burst cells within the ipsilateral PPRF. These cells are important during fixation and smooth pursuit. Abnormalities of these cells lead to opsoclonus and ocular flutter.
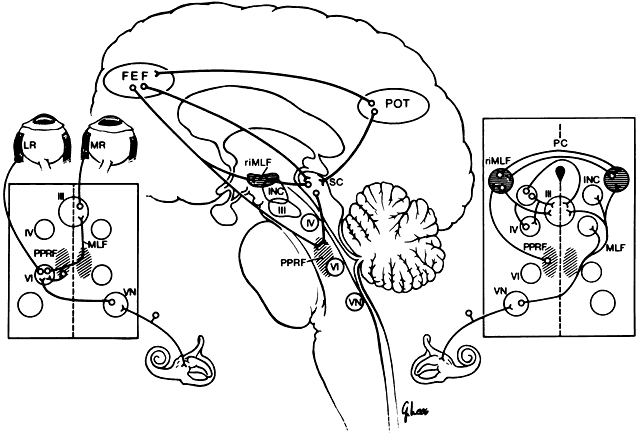
Lesions of the abducens nucleus cause an ipsilateral gaze palsy. In the rare instance in which there is an isolated lesion of the PPRF, there is an inability to make ipsilateral saccades. However, the response to vestibular stimuli (e.g., oculocephalic testing, caloric) and pursuit will be preserved. This is because there is a direct connection from the contralateral medial vestibular nuclei directly to the abducens nucleus, bypassing the PPRF (Fig. 2). These fibers synapse on abducens motor neurons and interneurons to the contralateral medial rectus muscle. Thus, labyrinthine reflex and pursuit eye movements may continue to be generated.
MEDIAL LONGITUDINAL FASCICULUS
The medial longitudinal fasciculus (MLF) is a fiber tract that extends from the spinal cord to the oculomotor nerve nucleus. It contains primarily ascending fibers, the majority of which arise in the superior and medial vestibular nuclei. The MLF is in close proximity to the ocular motor nuclei and influences both ipsilateral and contralateral nuclei. An abnormality of the MLF causes problems with horizontal and vertical gaze coordination of the two eyes. The clinically most important connection passing through the MLF links the contralateral abducens nucleus with the ipsilateral medial rectus subnucleus. Abnormalities of this tract produce an internuclear ophthalmoplegia. Such a lesion produces slowed or complete loss of adduction of the ipsilateral eye and abducting nystagmus of the fellow eye.
ROSTRAL INTERSTITIAL NUCLEUS OF THE MEDIAL LONGITUDINAL FASCICULUS
The rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) is located in the mesencephalon at the rostral termination of the MLF. This nucleus includes cells from the interstitial nucleus of Cajal. The riMLF has connections to motor neurons in the oculomotor and trochlear nuclei, as well as to the PPRF. On the basis of experimental and pathologic studies, this group of cells appears to be the immediate premotor area for vertical eye movements, both upward and downward. Its function is thus analogous to the PPRF for vertical eye movements. Damage to this area generally causes more difficulty with downward movement than with upward movement.
POSTERIOR COMMISSURE
Dorsal and rostral to the riMLF is the posterior commissure, a fiber tract that contains some scattered neuronal cell bodies. Lesions in this region produce abnormalities of upward gaze. It is likely that the fibers for upward gaze leave the riMLF and pass through this region before reaching the oculomotor and trochlear nuclei. Involvement of the posterior commissure may be part of the dorsal midbrain syndrome (Parinaud Syndrome). In this syndrome, there is impairment of upwardly directed saccades or, in extreme cases, loss of all vertical movement. Other signs include pupillary mydriasis and light-near pupillary dissociation, corectopia, and convergence-retraction nystagmus.
SUPERIOR COLLICULUS
These structures in the dorsal midbrain play a role in both ocular motor and sensory function. The superior colliculus receives visual input directly from branches of retinal ganglion cell axons. Visual input also comes indirectly from the visual cortex, the parietal and frontal lobes, and the substantia nigra. There are efferent projections to the brain-stem premotor areas. The superior colliculus can generate visually directed saccades independently and may play a role in the control of pursuit eye movements. In primates, ablation of both FEFs and both superior colliculi is necessary to produce permanent saccadic defects.
CEREBELLUM
The cerebellum appears to be involved in the immediate modulation of ongoing eye movements, as well as in the long-term adaptive processes that compensate for ocular motor dysmetria. The cerebellum controls and adjusts the size of saccades. The latter ability is essential for maintaining accurate ocular motor performance during growth and aging, during and after ocular motor disease, or even while using spectacles. For instance, the use of anisometropic spectacles produces a varying anisophoria in different directions of gaze, which must be compensated in each direction of gaze.
Hemicerebellectomy produces ipsilateral saccadic and contralateral pursuit defects, while total cerebellectomy creates persistent saccadic dysmetria and abolishes smooth pursuit. The cerebellum has numerous connections to nuclear and supranuclear ocular motor centers.
VESTIBULAR SYSTEM
The vestibular system consists of the semicircular canals, otolith organs, and vestibular nuclei. The first two structures form the peripheral vestibular system (labyrinth). There are three semicircular canals: horizontal (lateral), anterior (superior), and posterior (vertical). All are filled with perilymph, a fluid that communicates with the subarachnoidspace. The semicircular canals respond to angular acceleration produced during head rotation. The otoliths (the saccule and the utricle) respond to linear acceleration of the head and are especially important in maintaining eccentric eye position in response to a sustained head tilt. The neurosensory elements of the labyrinth are connected to sensory cells. The sensory cell bodies are located in the vestibular ganglion close to the peripheral vestibular apparatus. The vestibular nerve courses through the internal auditory meatus to enter the subarachnoid space. The nerve enters the brain stem in the superior portion of the medulla to reach the vestibular nuclei.
The vestibular nuclear complex is located in the medulla beneath the floor of the fourth ventricle. These nuclei are widely connected with nuclei in the brain stem, cerebral cortex, cerebellum, and reticular formation. Labyrinthine-stimulated eye movements are modulated by connections with the reticular formation and the cerebellum.
In addition to the labyrinthine inputs to the vestibular nuclei, visual and proprioceptive information reaches these nuclei. It is believed that opto-kinetic inputs from the striate cortex reach thevestibular nuclei by an accessory optic pathway. Proprioceptor input comes from the neck, providing the basis for cervico-ocular reflex. Each reflex acts to stabilize the orientation of the eyes in response to movements of the body and therefore opposes shifts of the line of sight caused by changes in position of the head or body.
Vertical gaze is produced by fibers carrying excitatory impulses from the ipsilateral vestibular nuclei. These pass to the contralateral side and ascend in the MLF to form a synapse in the appropriate ocular motor subnuclei. In addition, inhibitory projections from the same canals ascend ipsilaterally to mediate relaxation of the antagonist muscle or muscles (see Fig. 2; right inset).
Horizontal gaze produced by the vestibular system is by a direct excitatory projection to the contralateral abducens nucleus (see Fig. 2; left inset). The ipsilateral medial rectus muscle is innervated by way of an intercalated abducens neuron, as well as by some direct fibers to the medial rectus subnucleus. Each labyrinth exerts a continuous tonic innervation attempting to turn and rotate the eye to the opposite side. For example, the right labyrinth produces levoversion and levocycloversion. Removal of one labyrinth results in the eyes being turned conjugately toward that side because of the unopposed, intact labyrinth. Although the tonus from the labyrinth is constantly present, it may be superseded by other sources of ocular control. In particular, the visual ocular reflex often overrides thevestibular reflex. The visual ocular reflex attempts to ensure continued stimulus stabilization on the fovea.
Movements initiated by the otolith apparatus tend to keep the eyes fixed in position after a change in the position of the head. The otolith system generally attempts to keep the eyes aligned with the horizontal meridian. They do not appear to be involved in horizontal eye movements. The magnitude of the cyclovertical response is considered to be one-tenth of the head tilt. For every 10 degrees of head tilt, the eyes cyclovert 1 degree in the opposite direction. The maximal torsional response is produced by approximately 60 degrees of head tilt.
An important vestibular response in humans is the dynamic eye response produced by the semicircular canals. This system repositions the eyes during acceleration and deceleration of the head. The endolymph within the semicircular canals is displaced when the head is moved. This results in a change in pressure on the ciliated cells of the crista ampullaris, resulting in a stimulus to the brain. Once the head movement reaches a stable unchanging velocity, the pressure gradient disappears, and the peripheral vestibular signal disappears 30 to 45 seconds later. Thus, the semicircular canals make no contribution to the maintenance of static ocular position.
The semicircular canals function in a reciprocal fashion so that when an anterior canal is stimulated, the posterior canal is inhibited. Each canal primarily drives two extraocular muscles, one in each eye, that rotate the globe in the same plane as that in which semicircular canal is oriented. The horizontal canal excites the ipsilateral medial rectus muscle and the contralateral lateral rectus muscle. The anterior canal excites the ipsilateral superior rectus muscle and the contralateral inferior oblique muscle. The posterior canal excites the ipsilateral superior oblique muscle and the contralateral inferior rectus muscle.
A simple method for assessing the vestibulo-ocular reflex uses a direct ophthalmoscope. While the physician views one optic nerve, the patient's face is moved gently from side to side. If the vestibulo-ocular reflex is normal, the optic disk remains stationary. The vestibulo-ocular system also may be tested by active rotation of the head, called the doll's head maneuver. Such rotation stimulates a vestibular compensatory eye movement, allowing the clinician to evaluate the integrity of brain-stem extraocular muscle control. Disorders of the vestibulo-ocular reflex are nearly always manifest by oscillopsia.
CEREBRAL CORTEX
The most important centers for visually directed control of eye movements in humans are in the cerebral cortex. These include the FEFs and the cortex at the parieto-occipital-temporal (POT) junction.
FRONTAL EYE FIELDS
The frontal eye fields (FEF) are located at Brodman's area 8, the posterior end of the second frontal convolution. The FEFs are important for the generation of vertical and horizontal saccades. There are at least three different pathways from the FEF to the brain stem (see Fig. 2). First, the ventral pathway projects by way of the posterior portion of the anterior limb of the internal capsule and the medial part of cerebral peduncle to reach the pons, where there is a partial decussation and termination in the PPRF. Second, the dorsal pathway passes from the FEF through the thalamus, the pulvinar, the pretectal nuclei, and the superior colliculus to reach the brain stem. Third, the intermediate pathway extends from the FEF to the rostral ocular motor nuclei and the interstitial nucleus of Cajal. Although there are ipsilateral and contralateral projections, the predominant projections from the FEF to both the PPRF and the riMLF appear to be contralateral.
MIDDLE TEMPORAL AREA
The cerebral cortex in the region of the POT junction is important in the control of smooth pursuit eye movements and object tracking in space. This area is known as the middle temporal (MT) area in nonhuman primates. The area of the human brain that is the equivalent of the MT cortex of the nonhuman primate is Flechsig's area 10. The MT receives visual information from the striate and prestriate cortex. It projects to the brain stem, cerebellum, superior colliculi, and the FEF. The latter projections modulate visually directed saccadic eye movements. The POT junction cortex plays the key supranuclear role in the visual ocular reflex by way of projections to the PPRF and riMLF. This reflex keeps a moving image projecting on the fovea. There are specific efferent fibers for horizontal, vertical, and torsional movements.
Damage to only one side of the MT cortex slows ipsilateral slow pursuit, requiring catch-up saccades. Such lesions also temporarily impair pursuit responses to fast targets in moving in either direction.