|
|
Chapter 24: Lasers in Ophthamology
Author:
Lasers in Ophthamology
Ophthalmology was the first medical specialty to utilize laser energy in patient treatment, and it still accounts for more laser operations than any other specialty. The transparency of the optical media allows laser light to be focused upon the intraocular structures without the need for endoscopy. Laser therapy has made the treatment of a number of serious ocular diseases much safer and more effective. Lasers are also used to alter the refractive state of the eye and to perform cosmetic surgery upon the eyelids. Low-energy scanning laser systems are useful for diagnostic imaging of ocular structures and for measuring blood flow by interferometry. Because laser surgery irreversibly changes tissue, ocular laser surgery should be performed only by ophthalmologists with laser experience.
OCULAR LASER SYSTEMS
A laser consists of a transparent crystal rod (solid-state laser) or a gas- or liquid-filled cavity (gas or fluid laser) constructed with a fully reflective mirror at one end and a partially reflective mirror at the other. Surrounding the rod or cavity is an optical or electrical source of energy that will raise the energy level of the atoms within the rod or cavity to a high and unstable level, a process known as population inversion. When the excited atoms spontaneously decay back to a lower energy level, their excess energy is released in the form of light. This light can be emitted in any direction. In a laser cavity, however, light emitted in the long axis of the cavity can bounce back and forth between the mirrors, setting up a standing wave that stimulates the remaining excited atoms to release their energy into the standing wave, producing an intense beam of light that exits the cavity through the partially reflective mirror. The light beam produced is all of the same wavelength (monochromatic), with all of the light waves in phase with each other (coherent). The light waves follow closely parallel courses with almost no tendency to spread out. These unique properties of laser light allow the beam to be focused down to extremely small spots with very high energy densities. The laser light energy can be emitted continuously or in pulses, which may have pulse durations of nanoseconds or less.
MECHANISMS OF LASER EFFECTS
Photocoagulation
The principal lasers used in ophthalmic therapy are the thermal lasers, in which tissue pigments absorb the light and convert it into heat, thus raising the target tissue temperature high enough to coagulate and denature the cellular components. These lasers are used for retinal photocoagulation, for treatment of diabetic retinopathy (Figure 24-1) and sealing of retinal holes, and for photocoagulation of the trabecular meshwork, iris, and ciliary body in the treatment of glaucoma. They can be used at higher energy levels to evaporate tissue, as in laser iridotomy. These laser photocoagulators operate in continuous mode or very rapidly pulsed (thermal) mode. The (blue)-green argon laser is the workhorse of this class. Others include the krypton red laser; the solid-state diode laser, producing a near infrared wavelength; the tunable dye laser, producing wavelengths from green to red; the frequency-doubled Nd:YAG laser, producing green light; and the thermal mode Nd:YAG laser, producing infrared light. Because laser light is monochromatic, selective absorption into specific tissues by specific wavelengths is possible, while adjacent tissues are spared. An example is the yellow wavelength of the tunable dye laser, which can be used to treat neovascularization near the macula because the yellow light is absorbed by hemoglobin but not by the yellow xanthophyll pigment of the macula. Absorption of laser light by specific tissues can be enhanced by intravenous injection of absorbing dyes such as fluorescein or indocyanine green.
Photodisruption
Photodisruption lasers release a giant pulse of energy with a pulse duration of a few nanoseconds. When this pulse is focused to a 15-25 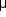 m spot, so that the nearly instantaneous light pulse exceeds a critical level of energy density, "optical breakdown" occurs in which the temperature rises so high (about 10,000 °K) that electrons are stripped from atoms, resulting in a physical state known as a plasma. This plasma expands with momentary pressures as high as 10 kilobars (150,000 psi), producing a cutting effect upon the ocular tissues. Because the initial plasma size is so small, it has little total energy and produces little effect away from the point of focus. Though a significant shock wave is produced, studies on polyethylene membranes indicate that direct contact with the plasma is required for cutting tissue. Photodisruptors are used principally for perforating cloudy posterior capsules after cataract extraction and for performing laser iridotomy. The principal laser of this class is the Q-switched neodymium:YAG laser.
m spot, so that the nearly instantaneous light pulse exceeds a critical level of energy density, "optical breakdown" occurs in which the temperature rises so high (about 10,000 °K) that electrons are stripped from atoms, resulting in a physical state known as a plasma. This plasma expands with momentary pressures as high as 10 kilobars (150,000 psi), producing a cutting effect upon the ocular tissues. Because the initial plasma size is so small, it has little total energy and produces little effect away from the point of focus. Though a significant shock wave is produced, studies on polyethylene membranes indicate that direct contact with the plasma is required for cutting tissue. Photodisruptors are used principally for perforating cloudy posterior capsules after cataract extraction and for performing laser iridotomy. The principal laser of this class is the Q-switched neodymium:YAG laser.
Photo-evaporation
The prototype of this class is the carbon dioxide laser, which produces a long-wavelength infrared heat beam. The beam is absorbed by water and therefore will not enter the interior of the eye. This laser can evaporate away surface lesions such as lid tumors and can be used for bloodless incisions in skin or sclera. The carbon dioxide laser beam can also be delivered through probes for contact photo-incision and photocoagulation within the eye. Used in a rapidly pulsed mode, this laser produces a controlled superficial skin burn that can tighten the eyelid skin for cosmetic improvement. The erbium and holmium lasers produce similar effects.
Photodecomposition
Photodecomposition lasers produce very short wavelength ultraviolet light that interacts with the chemical bonds of biologic materials, breaking the bonds and converting biologic polymers into small molecules that diffuse away. These lasers collectively are called excimer ("excited dimer") lasers because the cavity contains two gases, such as argon and fluorine, that react into unstable molecules which then emit the laser light. They can precisely recontour the corneal surface by computer-controlled ablation of successive thin layers of the cornea, correcting refractive errors such as myopia, hyperopia, and astigmatism. Photodecomposition lasers can also remove shallow corneal opacities resulting from injuries or dystrophies.
THERAPEUTIC APPLICATION OF LASERS
DIABETIC RETINOPATHY
In nonproliferative diabetic retinopathy, vision may be impaired by macular edema and exudates resulting from breakdown of the inner blood-retinal barriers at the level of the retinal capillary endothelium. Many patients with long-term diabetes mellitus will gradually develop diffuse obliteration of the retinal microcirculation, especially of the capillaries, resulting in generalized retinal ischemia. This ischemic state leads to neovascularization of the retina and iris, at least partly mediated by diffusible vasoproliferative factors released from the ischemic retina into the ocular fluids. Untreated retinal neovascularization leads to vitreous hemorrhages and traction retinal detachment. Iris neovascularization produces neovascular glaucoma. (The clinical features of diabetic retinopathy are more fully discussed in Chapter 10.)
Diabetic macular edema is treated by focal or grid pattern laser photocoagulation, which principally acts by augmenting the function of the retinal pigment epithelium. Burns 50-100 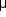 m in diameter are applied, avoiding the foveal avascular zone, which is approximately 300
m in diameter are applied, avoiding the foveal avascular zone, which is approximately 300 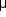 m in diameter. The areas of leakage to be treated can be identified by fluorescein angiography (areas of discrete or diffuse fluorescein leakage and areas of capillary nonperfusion associated with retinal thickening) or by clinical examination (zones of retinal thickening). The most effective treatment for retinal and iris neovascularization is panretinal photocoagulation (PRP), which usually consists of treating the entire retina-except for the area within the temporal vascular arcades-with 200-500
m in diameter. The areas of leakage to be treated can be identified by fluorescein angiography (areas of discrete or diffuse fluorescein leakage and areas of capillary nonperfusion associated with retinal thickening) or by clinical examination (zones of retinal thickening). The most effective treatment for retinal and iris neovascularization is panretinal photocoagulation (PRP), which usually consists of treating the entire retina-except for the area within the temporal vascular arcades-with 200-500 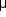 m diameter burns placed one to two burn widths apart. PRP requires a total of at least 2000 and sometimes 6000 or more burns, usually delivered over two or more sessions spaced about 2 weeks apart. Retrobulbar or peribulbar anesthesia is sometimes required, particularly if areas of the retina need to be re-treated because of recalcitrant or recurrent neovascularization. Treatment is staged to reduce the incidence of uveitis, macular edema, exudative retinal detachment, and even shallowing of the anterior chamber with secondary angle closure. In the presence of significant macular edema, focal macular photocoagulation should be carried out prior to or together with the PRP to avoid increase in edema.
m diameter burns placed one to two burn widths apart. PRP requires a total of at least 2000 and sometimes 6000 or more burns, usually delivered over two or more sessions spaced about 2 weeks apart. Retrobulbar or peribulbar anesthesia is sometimes required, particularly if areas of the retina need to be re-treated because of recalcitrant or recurrent neovascularization. Treatment is staged to reduce the incidence of uveitis, macular edema, exudative retinal detachment, and even shallowing of the anterior chamber with secondary angle closure. In the presence of significant macular edema, focal macular photocoagulation should be carried out prior to or together with the PRP to avoid increase in edema.
Adequate PRP is highly effective in producing regression of neovascularization. The exact mechanism of action has not been established, but reduction in the degree of retinal ischemia and production of diffusible vasostimulative substances are thought to be important. The type of laser used does not appear to influence the efficacy of PRP, but particular characteristics can be important in treatment, eg, the easier use of the diode infrared laser in the presence of vitreous hemorrhage. Direct treatment of retinal neovascularization is rarely necessary but can be performed if there are residual new vessels after extensive PRP, particularly if they are responsible for vitreous hemorrhage.
PRP does not cause regression of the fibrosis associated with retinal neovascularization, which is responsible for tractional retinal detachment. Furthermore, PRP can be precluded by vitreous hemorrhage. Thus, PRP should be undertaken as soon as high-risk clinical features have developed: optic disk neovascularization, retinal neovascularization at other sites associated with vitreous hemorrhage, and iris neovascularization. Retinal neovascularization away from the optic disk without vitreous hemorrhage may be treated either by scatter photocoagulation limited to the adjacent areas of retina or by PRP, particularly if there is advanced or rapidly proliferative disease in the other eye. Because timely PRP is so effective in preventing blindness in diabetes, any diabetic with retinopathy greater than scattered microaneurysms should be seen on a regular basis by an ophthalmologist.
CENTRAL RETINAL VEIN THROMBOSIS
Thrombosis involving the central retinal vein produces the classic fundus appearance of disk swelling, marked venous dilation, and almost confluent retinal hemorrhages (see Chapter 10). While these changes can progress to retinal neovascularization, vitreous hemorrhage, and fibrosis, a more common complication is the development of rubeosis iridis with neovascular glaucoma. If severe retinal ischemia is present on fluorescein angiography, there is a 60% chance of this complication. In neovascular glaucoma, substances produced by the ischemic retina diffuse forward and stimulate formation of a fibrovascular membrane that grows across the iris surface and covers the trabecular meshwork, resulting in glaucoma characterized by very high pressure, pain, and marked resistance to medical and surgical therapy, so that enucleation of the blind and painful eye may be required. PRP as described above for treatment of proliferative diabetic retinopathy-preferably with the krypton red or diode infrared laser to avoid preretinal fibrosis caused by heat absorption in the hemorrhages-can greatly reduce the incidence of neovascular glaucoma in ischemic central retinal vein thrombosis. It is most effectively applied when iris neovascularization is present but before neovascular glaucoma has developed. However, in clinical practice this timing can be difficult to achieve. Once neovascular glaucoma is present, adequate panretinal photocoagulation will usually cause regression of the anterior segment neovascularization, allowing the glaucoma to be controlled medically or by filtering surgery. Unfortunately, established neovascular glaucoma is often associated with corneal edema, miosis, or hyphema so that PRP cannot be performed and only cyclophotocoagulation or enucleation can be used. For this reason, prophylactic PRP may be advisable in all cases of ischemic central retinal vein thrombosis. A relative afferent pupillary defect, vision of 20/200 or less, and multiple retinal cotton-wool spots are highly suggestive of ischemia severe enough to warrant prophylactic PRP. Electroretinography and fluorescein angiography provide further evidence when needed.
BRANCH RETINAL VEIN THROMBOSIS
This condition varies from localized areas of venous congestion and hemorrhage to hemiretinal involvement from thrombosis of the superior or inferior division of the central retinal vein. The principal complications are chronic macular edema (with or without exudates) and retinal neovascularization followed by vitreous hemorrhage and traction retinal detachment. When the area of ischemic retina is demonstrated by fluorescein angiography to exceed five disk diameters in extent, prophylactic scatter photocoagulation of the ischemic area can be performed to reduce the risk of retinal neovascularization, which usually develops in the region of the retinal vascular arcades. If retinal neovascularization does develop, laser treatment should be performed promptly, preferably before vitreous hemorrhage occurs. Focal and grid-pattern argon green laser photocoagulation is used to treat macular edema and exudates by obliteration of areas of retinal leakage as demonstrated by fluorescein angiography.
RETINAL TEARS
When a peripheral retinal tear occurs-usually due to senile vitreous degeneration causing vitreous traction-the patient often notices the sudden appearance of dot-like floaters. The tear can cause retinal detachment, but if detected prior to the accumulation of subretinal fluid it can be walled off by applying a double ring of laser burns around it to create an adhesion of the adjacent attached retina to the pigment epithelium. Once retinal detachment has occurred, surgery is required. Prompt retinal examination through a dilated pupil is therefore indicated in any eye with sudden onset of floaters, particularly dot-like floaters suggesting red blood cells.
MACULAR DEGENERATION & RELATED DISEASES
Bruch's membrane forms a barrier layer between the retinal pigment epithelium and the choriocapillaris, which is the capillary layer of the choroid. If Bruch's membrane deteriorates or is damaged, capillary nets can grow through the break beneath the pigment epithelium, first causing exudative pigment epithelial detachment with distortion and edema of the overlying retina and later causing hemorrhage and fibrosis with destruction of retinal function in that area. The macular retina is particularly likely to develop Bruch's membrane breaks and neovascularization, though these changes can occur anywhere in the fundus. The most frequent cause is age-related macular degeneration, which begins as asymptomatic yellowish deposits (drusen) in the macular area. As the years advance, pigment epithelial atrophy and clumping are seen, and finally Bruch's membrane breaks appear, leading to fluid leaks, neovascularization, fibrosis, and loss of central vision. This condition is the leading cause of legal blindness in the older population. Bruch's membrane breaks and neovascular nets can occur at sites of old chorioretinitis from childhood histoplasmosis, toxoplasmosis, and various other inflammatory disorders. They can develop from traumatic choroidal ruptures, even in children, and can occur in a host of hereditary diseases involving the retina. If sub-pigment epithelial neovascular nets are located away from the central foveal area, they can be destroyed by careful laser photocoagulation to preserve central vision. The yellow macular pigment (xanthophyll) strongly absorbs blue light, weakly absorbs green light, and does not absorb yellow, orange, or red light. Hemoglobin strongly absorbs blue, green, yellow, and orange light but very weakly absorbs red light. Melanin absorbs all visible wavelengths. Selective absorption of laser energy is therefore possible. If the neovascular net has melanin pigment in it or is bleeding, then krypton red laser light allows deep penetration to the choriocapillaris without hemoglobin or xanthophyll absorption. If the net does not have much melanin and has not bled, argon green or dye laser yellow or orange will be absorbed by hemoglobin to coagulate the net but the scattered light will not be absorbed by xanthophyll. The whole neovascular net must be heavily treated for control. Unfortunately, in many cases the net is already under the fovea at the time of diagnosis, or bleeding is already so extensive that laser treatment is not possible. Early diagnosis is therefore of utmost importance in this group of diseases, and patients at risk must diligently look for and report the small blurs and distortions of vision that are the first signs of neovascular growth. Fluorescein angiography can then be used to demonstrate the retinal circulation, including areas of neovascularization and abnormal vascular permeability. Direct laser treatment of subfoveal neovascular membranes produces an immediate permanent reduction in central acuity but may lead to a better long-term outcome than can be expected without treatment.
GLAUCOMA
Treatment of open-angle glaucoma, angle-closure glaucoma, and glaucoma resistant to surgery has been radically altered by availability of effective laser techniques.
Angle-Closure Glaucoma
In primary angle-closure glaucoma, aqueous flow through the pupil is blocked by contact of the lens with the posterior surface of the iris. The resulting pressure in the posterior chamber forces the peripheral iris forward into contact with the trabecular meshwork, blocking outflow and increasing intraocular pressure. While the classic, dramatic acute glaucoma attack is usually considered the prototype of angle-closure glaucoma, acute attacks are actually very rare. Creeping or subacute angle-closure glaucoma is far more common, especially in darkly pigmented eyes, and can occur with a normal central anterior chamber depth. Angle closure can be determined only by examining the angle, which is usually done by slitlamp gonioscopy through a gonioscopy contact lens containing a mirror. Because angle closure is the commonest type of glaucoma in Asian populations, it is probably the most common type of glaucoma worldwide. Surgical iridectomy was the standard treatment for angle-closure glaucoma for decades but carried the risks of hemorrhage, infection, anesthetic accidents, and even sympathetic ophthalmia. Studies of ruby laser iridotomy began in animals in 1964, but not until 1975 was an effective argon laser technique developed for human eyes. In 1979, laser iridotomy was made more effective by the Abraham contact lens, whose 66-diopter focusing button increased iris energy density. The more recent Wise iridotomy-sphincterotomy lens has a 103-diopter button that gives the highest energy density possible with a practical contact lens. With these high-energy densities, laser iridotomy (Figure 24-2) is nearly 100% successful with either the argon laser or the Q-switched Nd:YAG laser, failing only when the cornea is so cloudy that the laser cannot be focused upon the iris. With the argon laser, the beam is focused through the Wise lens upon the far peripheral iris fibers, which are cut in a line parallel to the limbus by multiple shots at 0.01-s or 0.02-s exposures and energy levels of 1-2 W. With the Nd:YAG laser, iridotomy can be done through the Wise lens by a high-power single-point method using about 8 mJ per shot in a single-shot or a two- or three-shot burst, or it can be done by cutting the far peripheral iris fibers in a line parallel to the limbus with multiple shots at 1-1.5 mJ. The argon laser is preferable for dark brown, thick irides, which tend to bleed with the Nd:YAG laser, while light blue irides do not absorb argon laser energy well and are more easily perforated with the Nd:YAG laser. If both lasers are available, a very efficient method for thick brown irides is to cut the thick stroma with the argon laser and then remove strands and pigment with a few low-power Nd:YAG laser bursts. Because of its safety, laser iridotomy should be done not only for established angle-closure glaucoma but whenever progressive pupillary block is occurring, before irreversible damage from angle closure has occurred.
Primary Open-Angle Glaucoma
This is the most common type of glaucoma in Western countries and is characterized by painless gradual reduction in trabecular meshwork function with decreasing outflow, increasing intraocular pressure, progressive cupping of the optic nerve, and insidious loss of visual field, leading ultimately to blindness. Topical medical therapy is the standard approach. If medical therapy is not adequate, laser trabeculoplasty is usually the next therapy (Figure 24-3). This consists of spacing 100 or more nonperforating argon laser burns 360 degrees around the trabecular meshwork to shrink the collagen in the tissues of the trabecular ring, reducing the circumference and therefore the diameter of the trabecular ring, pulling the trabecular layers apart with reopening of the intertrabecular spaces and of Schlemm's canal. Growth of new trabecular cells may also occur. Trabeculoplasty increases outflow and has no influence upon aqueous secretion. Though in some eyes the abnormal meshwork can continue to deteriorate, with late failure requiring filtration surgery, 10-year control of glaucoma has been reported. Most eyes continue to require some medical therapy. The value of trabeculoplasty lies in reducing medical therapy and postponing or avoiding the risks of filtration surgery. The only significant side effects are a rise in pressure for 1-4 hours in about one-third of eyes (preventable by apraclonidine drops) and a rise in pressure for 1-3 weeks in about 2% of treated eyes. To reduce the severity of these pressure rises, many laser surgeons do trabeculoplasty with 50 laser burns in 180 degrees of the trabecular meshwork, reserving the other 180 degrees for treatment later if necessary. Trabeculoplasty with other laser wavelengths, such as green, yellow, red, and infrared, is also effective. In a large randomized trial, primary argon laser trabeculoplasty gave better control of open-angle glaucoma than did primary medical therapy alone.
Cyclophotocoagulation
Glaucoma refractory to the usual operative procedures can often be controlled by direct destruction of the ciliary processes. This was first done by diathermy and later by cryosurgery. Cyclophotocoagulation through intact conjunctiva and sclera was originated by Beckman, using a high-energy ruby laser, but is currently performed by contact delivery through a fiberoptic probe with the thermal-mode Nd:YAG laser or the diode laser (Figure 24-4). Good control is usually obtained, but multiple treatments may be required. Side effects such as pain, inflammation, and reduction of vision are significantly less severe than with cryosurgery. Laser endocyclophotocoagulation can be performed using a fiberoptic probe passed through the pars plana during vitrectomy.
Laser Suture Lysis
Trabeculectomy is currently the procedure of choice for glaucoma drainage surgery (see Chapter 11) because the partial-thickness scleral flap reduces the incidence of complications caused by early postoperative hypotony. In order to increase the degree of drainage and perhaps achieve greater long-term reduction in intraocular pressure-similar to that obtained with the older full-thickness drainage procedures-laser lysis of the scleral flap sutures can be carried out 7-14 days after standard trabeculectomy and 3-8 weeks after trabeculectomy augmented by antifibrotic therapy with mitomycin. The black 10-0 nylon sutures are cut by focusing short laser pulses upon them through the transparent conjunctiva, aided by compressing the overlying tissues with the Hoskins suture lens. The argon laser may be used, but if hemorrhage is present the krypton red or diode infrared laser is preferred to avoid flap perforation by hemoglobin absorption of argon blue-green laser wavelengths.
LASER PHOTOMYDRIASIS & LASER SPHINCTEROTOMY
For a variety of reasons, but most frequently because of long-term miotic therapy, the pupil can become fixed at a very small size, reducing vision and interfering with pupillary dilation for retinal examination or treatment. Multiple laser burns of 200  m diameter placed on the iris in a ring outside the pupil will produce temporary enlargement, but marked uveitis and intraocular pressure rises can occur and the dilating effect disappears over time. A better method is argon laser sphincterotomy, in which one or more linear cuts across the iris sphincter are made by focusing the argon laser through the Wise iridotomy-sphincterotomy lens, using numerous shots at 0.01-s exposure and about 1 W energy. This produces permanent enlargement of the pupil with less irritation. Energy levels must be kept low to avoid lens burns. The Nd:YAG laser at very low energy levels can be used to cut persistent nonpigmented bridging strands.
m diameter placed on the iris in a ring outside the pupil will produce temporary enlargement, but marked uveitis and intraocular pressure rises can occur and the dilating effect disappears over time. A better method is argon laser sphincterotomy, in which one or more linear cuts across the iris sphincter are made by focusing the argon laser through the Wise iridotomy-sphincterotomy lens, using numerous shots at 0.01-s exposure and about 1 W energy. This produces permanent enlargement of the pupil with less irritation. Energy levels must be kept low to avoid lens burns. The Nd:YAG laser at very low energy levels can be used to cut persistent nonpigmented bridging strands.
POSTERIOR CAPSULOTOMY AFTER CATARACT SURGERY
Modern cataract surgery uses extracapsular extraction or phacoemulsification followed by posterior chamber intraocular lens (IOL) implantation (see Chapter 8). If the posterior capsule supporting the IOL later opacifies, vision can be restored by focusing Q-switched Nd:YAG laser pulses just posterior to the capsule to produce a central capsulotomy (thus avoiding further intraocular surgery). Careful focus through a condensing contact lens is necessary to avoid damage to the IOL. A small increase in the risk of retinal holes and retinal detachment is present after capsulotomy. Opacification of the capsule is not preventable at present, so that some eyes will require capsulotomy for useful vision. However, the risk of retinal detachment from delaying capsulotomy until vision is actually impaired is almost certainly less than the risk from primary capsulotomy during cataract surgery, which also carries the additional risks of IOL malposition and vitreous complications.
CUTTING VITREOUS BANDS & OPACITIES
Incomplete clearance of vitreous from the anterior chamber during the management of vitreous loss secondary to trauma or surgery may result in pupillary distortion, chronic uveitis, and cystoid macular edema. These bands can be cut with the Q-switched Nd:YAG laser, either directly through the cornea by focusing on the band through a condensing contact lens such as the Wise lens, or in the angle by focusing through the mirror of a condensing goniolens such as the Trokel lens or the Lasag color graphics adapter (CGA) lens. Multiple shots at minimal optical breakdown levels should be used to minimize concussion to cornea and iris. Eyes with chronic cystoid macular edema have improved after cutting of vitreocorneal bands. Localized opacities and bands in the anterior vitreous can be cut with the Nd:YAG laser to clear the visual axis or reduce traction upon the retina. Much of the time, however, such vitreous abnormalities are widespread, and surgical vitrectomy is required.
VAPORIZATION OF LID TUMORS
The carbon dioxide laser has been used to bloodlessly remove both benign and malignant lid tumors. However, because of scarring, lack of a histologic specimen, and inability to assess margins, laser treatment for this purpose appears inferior to surgery in most cases.
REFRACTIVE SURGERY
The excimer lasers, particularly the 193-nm wavelength argon fluoride laser, can evaporate tissue very cleanly with almost no damage to cells adjacent to or under the cut. By using multiple pulses and progressively changing spot size to evaporate successive thin layers of the cornea, computer-controlled recontouring of the cornea (PRK, photorefractive keratectomy) can precisely and it would seem permanently correct moderate myopic and astigmatic refractive errors (Figure 24-5). Initial difficulties with superficial corneal haze appear to have been overcome. Hyperopic and highly myopic (over 6 diopters) errors do not respond as well to PRK. Many thousands of eyes have been successfully treated for myopia in Europe, Asia, and the USA. Where available, PRK has largely replaced surgical radial keratotomy, which is less predictable and which is associated with complications, such as deep scarring, ocular perforation, intraocular infection, and late hyperopic shift, that do not occur with the laser. PRK does remove Bowman's membrane, to which the corneal epithelium adheres, which can sometimes produce corneal haze. To preserve this membrane, an alternative procedure commonly known by the acronym LASIK (laser in situ keratomileusis) consists of cutting a hinged lamellar flap of cornea with a mechanical keratome, performing the refractive laser ablation in the corneal bed, and then replacing the flap. Laser in situ keratomileusis (LASIK) has not so far been compared with PRK in prospective controlled studies.
COSMETIC LASER EYELID SURGERY
Exposing wrinkled eyelid skin to repeated 1 ms pulses from the carbon dioxide laser, obtained by rapid pulsing of the laser tube or by computer-controlled rapid scanning of a continuous small laser beam, evaporates the epidermis and induces collagen contraction in the dermis. When the epithelium regenerates, the skin is tightened and small wrinkles and crow's-feet are removed. The technique is more precise than older methods such as dermabrasion or chemical peels, but it still can sometimes be complicated by keloid scarring, hyperpigmentation, and herpesvirus infection. Surgeon experience is very important in obtaining good results. The erbium:YAG laser can be used in the same manner.
LASER DIAGNOSTIC IMAGING
Confocal imaging is a video method that uses a rapidly scanning tiny laser spot whose reflected light is imaged through a pinhole upon a detector, thus suppressing all reflections except those from the focal plane. By scanning at multiple levels and then combining the images by computer processing, precise and reproducible three-dimensional images of ocular structures can be produced. The principal use of these instruments is to evaluate and follow glaucoma-induced changes in the optic nerve head, but other uses include macular, lens, and corneal imaging. Laser interferometry is used to measure blood flow in the ciliary body and retinal blood vessels. Ocular coherence tomography can produce very high resolution optical sections of the cornea and retina to allow evaluation of diseases such as corneal dystrophies and macular degeneration.
REFERENCESList of Figures
| Figure 24-1: Argon laser burn scars in retina after panretinal photocoagulation for diabetic retinopathy. | |
| Figure 24-2: Laser iridotomy for angle-closure glaucoma. | |
| Figure 24-3: Laser trabeculoplasty. The laser is focused upon the trabecular meshwork to increase outflow. (Reproduced, with permission, from Schwartz A, et al: Argon laser trabecular surgery in uncontrolled phakic open- angle glaucoma. Ophthalmology 1981; 88: 205.) | |
| Figure 24-4: Laser cyclophotocoagulation. The laser light passes through the conjunctiva and sclera and is absorbed by the pigment in the ciliary body, producing thermal coagulation of secreting epithelium. | |
| Figure 24-5: Excimer laser photorefractive keratectomy. The laser cleanly photodecomposes corneal tissue in a controlled pattern to reshape the corneal curvature. (Photo courtesy of T Clapham, VISX Inc.); |
10.1036/1535-8860.ch24