|
Page 1 of 17
|
Print this Chapter ( K) |
Chapter 15: Ocular Disorders Associated With Systemic Diseases
Authors:
Ocular Disorders Associated With Systemic Diseases
Examination of the eye provides the ophthalmologist an opportunity to make a unique contribution to the diagnosis of systemic disease. Nowhere else in the body can a microcirculatory system be investigated with such precision, and nowhere else are the results of minute focal lesions so devastating. Many systemic diseases involve the eyes, and therapy demands some knowledge of the vascular, rheologic, and immunologic nature of these diseases.
VASCULAR DISEASE
NORMAL ANATOMY & PHYSIOLOGY
The blood supply to the eye is from the ophthalmic artery, which is the first branch of the internal carotid artery (see Chapter 1). The first branches of the ophthalmic artery are the central retinal artery and the long posterior ciliary arteries. The retina is perfused by retinal and choroidal vessels that provide contrasting anatomic and physiologic circulations. The retinal arteries correspond to arterioles in the systemic circulation. They function as end arteries and feed a capillary bed consisting of small capillaries (7 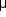 m) with tight endothelial junctions. Dependent on this anatomic arrangement is the maintenance of the blood-retina barrier, and this system is autoregulated, since there are no autonomic nerve fibers. Most of the blood within the eye, however, is in the choroidal circulation, which is characterized by a high flow rate, autonomic regulation, and an anatomic arrangement with collateral branching and large capillaries (30
m) with tight endothelial junctions. Dependent on this anatomic arrangement is the maintenance of the blood-retina barrier, and this system is autoregulated, since there are no autonomic nerve fibers. Most of the blood within the eye, however, is in the choroidal circulation, which is characterized by a high flow rate, autonomic regulation, and an anatomic arrangement with collateral branching and large capillaries (30 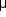 m), all of which have fenestrations in juxtaposition to Bruch's membrane. Examination of the retinal vessels is facilitated by the use of red-free light and fluorescein angiography, whereas indocyanine green angiography gives further information about the choroidal vessels.
m), all of which have fenestrations in juxtaposition to Bruch's membrane. Examination of the retinal vessels is facilitated by the use of red-free light and fluorescein angiography, whereas indocyanine green angiography gives further information about the choroidal vessels.
PATHOLOGIC APPEARANCES IN RETINAL VASCULAR DISEASE
Hemorrhages
Retinal hemorrhages result from diapedeses from veins or capillaries, and the morphologic appearances depend upon the size, site, and extent of damage to the vessel (Figure 15-1). Hemorrhages may be caused by any condition that alters the integrity of the endothelial cells. They usually indicate some abnormality of the retinal vascular system, and systemic factors should be considered in relation to (1) vessel wall disease (eg, hypertension, diabetes), (2) blood disorders (eg, leukemia, polycythemia), and (3) reduced perfusion (eg, carotid cavernous fistula, acute blood loss).
A. Preretinal Hemorrhages:
These result from damage to the superficial disk or retinal vessels and are usually large, producing a gravity-dependent fluid level.
B. Linear Hemorrhages:
These usually small hemorrhages lie in the superficial nerve fiber layers and hence have a characteristic linear appearance, conforming to the alignment of nerve fibers in any particular area of the fundus.
C. Punctate Hemorrhages:
Hemorrhages situated deeper in the substance of the retina are punctate and derived from capillaries and smaller venules. The circular appearance is related to the anatomic arrangement of structures in the retina.
D. Subretinal Hemorrhages:
These hemorrhages are less common because normally there are no blood vessels between the retina and the choroid. Such hemorrhages are large and red, with a well-defined margin and no fluid level. They are seen in relation to the disk and in any condition where abnormal vessels pass from the choroidal circulation into the retina.
E. Hemorrhages Under the Pigment Epithelium:
Hemorrhages situated under the pigment epithelium are usually dark and large, so that they must be differentiated from choroidal melanomas and hemangiomas.
F. White Central Hemorrhages (Roth's Spots):
Superficial retinal hemorrhages with pale or white centers are not pathognomonic of any disease process but may arise in a variety of circumstances: (1) retinal infarction (cotton-wool spot) with surrounding hemorrhage; (2) retinal hemorrhage in combination with extravasation of white corpuscles (eg, leukemia); and (3) retinal hemorrhage with central resolution.
Acute Ocular Ischemia
A. Optic Disk Infarction (Anterior Ischemic Optic Neuropathy):
Impairment of the blood supply to the optic disk produces sudden visual loss, usually with an altitudinal field defect and pallid swelling of the optic disk. The primary abnormality is complete or partial interruption of the choroidal blood supply to the disk, while the retinal capillaries on the surface of the disk appear dilated. Fluorescein angiography confirms the circulatory alterations (Figure 15-2). Pathologic studies show infarction of the retrolaminar region of the optic nerve. The explanation for the vulnerability of the short posterior ciliary vessels supplying this region is unknown. Optic disk infarction is often caused by giant cell arteritis in old age and by hypertension and arteriosclerotic disease in middle age. Small optic disks are particularly prone to infarction.
B. Choroidal Infarction:
This is extremely rare, though certain clinical appearances have been attributed to ciliary vessel occlusion. These include small pale areas in the equatorial region that resolve to leave mottled pigmentary areas (Elschnig's spots) due to necrosis of the pigment epithelium. Larger infarcts may occur and may be triangular or linear (Figure 15-3).
C. Retinal Infarction:
The funduscopic appearance of arteriolar occlusion depends on the size of the vessel occluded, the duration of occlusion, and the time course. Occlusion of major arterioles produces a total, hemispheric, or segmental pallid swelling of the retina. Occlusion of a precapillary retinal arteriole produces the pathognomonic appearance of a cotton-wool spot (Figure 15-4). This consists of a pale, slightly elevated swelling usually one-fourth to one-half the size of the optic disk. Pathologic examination shows distention of neurons, with cytoid bodies (Figure 15-5); electron microscopy shows the accumulation of axoplasm and organelles. Occlusion of arterioles, whether due to intrinsic vessel wall disease or to intramural factors, may produce these pathognomonic signs.
D. Transient Retinal Ischemia (Amaurosis Fugax):
Transient episodes of monocular visual loss lasting 5-10 minutes are characteristic of amaurosis fugax. Patients often describe a curtain coming down from above or across their vision, usually with complete return of vision within seconds or minutes. Paresthesias in the contralateral limbs localize the disorder to the carotid artery and suggest involvement of the ophthalmic artery and middle cerebral artery. It is important for the ophthalmologist to auscultate the carotid for a systolic bruit and to search the fundus for emboli. Amaurosis fugax is most commonly due to retinal emboli, of which there are three main types.
1. Cholesterol emboli-
These so-called Hollenhorst plaques usually arise from an atheromatous plaque in the carotid artery and consist of cholesterol and fibrin. They lodge at the bifurcation of retinal arterioles, are refractile, and may appear larger than the vessel that contains them (Figure 15-6).
2. Calcific emboli-
Originating from damaged cardiac valves, these emboli lodge within the arteriole, producing complete occlusion and infarction of the distal retina. Calcific emboli are solid and calcified and occur in younger patients with a variety of cardiac lesions.
3. Platelet-fibrin emboli-
Most cases of amaurosis fugax are probably due to the transit of platelet aggregates through the retinal and choroidal circulations. The emboli are usually broken up as they traverse the retinal circulation and hence are rarely seen, though occasionally they produce retinal infarction. Arising from abnormalities of the heart or great vessels, they may be reduced by drugs that reduce platelet aggregation (eg, aspirin).
Retinal emboli most commonly arise from carotid artery disease (see Chapter 14). A cardiac origin such as atrial fibrillation, mitral valve prolapse, or subacute infective endocarditis needs to be considered, particularly in patients under 40 years of age or those with a history of cardiac disease.
There are several other causes of amaurosis fugax, including factors that induce temporary reduction in ocular perfusion, eg, arterial disease, cardiac disorders, hematologic disorders, retinal or choroidal migraine, and, rarely, elevation of intraocular pressure (Table 15-1).
Central Retinal Vein Occlusion (![]() Figure 15-7)
Figure 15-7)
Central retinal vein occlusion is an important cause of visual morbidity in elderly people, particularly those with hypertension or glaucoma.
Figure 15-7: Central retinal vein occlusion. Left: Photograph shows linear hemorrhages in the nerve fiber layer and punctate hemorrhages in the deeper retinal layers. Right: Fluorescein angiogram shows dilation of the veins.
Fundus examination shows dilated tortuous veins with retinal and macular edema, hemorrhages all over the posterior pole, and cotton-wool spots. The arterioles are usually attenuated, indicating generalized microvascular disease.
The prognosis for vision is poor. Fluorescein angiography demonstrates two types of response: a nonischemic type, with dilation of retinal vessels and edema; and an ischemic type, with large areas of capillary nonperfusion or evidence of retinal or anterior segment neovascularization. In 93% of ischemic and 50% of nonischemic central retinal vein occlusions, the ultimate visual acuity is less than 20/200.
Central retinal vein occlusion has an increased incidence in certain systemic conditions such as diabetes mellitus, hypertension, collagen vascular diseases, and hyperviscosity syndromes (eg, Waldenström's macroglobulinemia, angioimmunoblastic lymphadenopathy). However, the prevalence of cerebrovascular or cardiovascular disease is not increased compared to the general population. Investigations include measurement of serum lipids, plasma proteins, plasma glucose, and assessment of blood viscosity by hemoglobin, hematocrit, and fibrinogen estimations. In young patients, protein C, activated protein C resistance, protein S, and antithrombin III levels should be measured to exclude abnormalities of the thrombolytic system. If hypertension is present, simple renal function tests, including urea and electrolytes, estimation of creatinine clearance, microscopic examination of the urine, and renal ultrasound are indicated.
Treatment of retinal vein occlusion is unsatisfactory. Trials with anticoagulants and fibrinolytic agents have not been successful. In ischemic central retinal vein occlusion, panretinal laser photocoagulation is effective in preventing and treating secondary neovascular glaucoma.
Occasionally, central retinal vein occlusion occurs in young people and may be associated with cells in the vitreous. Rheologic investigations are usually negative, and the prognosis for vision is good.
Retinal Branch Vein Occlusion (![]() Figure 15-8)
Figure 15-8)
Occlusion of a branch vein should be viewed as part of the spectrum of central retinal vein occlusion. Investigations are similar in the two conditions, but arterial disease-particularly hypertension-is common. Branch retinal vein occlusion occurs more frequently in the superotemporal and inferotemporal regions and particularly at sites where arteries cross over veins, and only rarely where veins cross over arteries.
Figure 15-8: Retinal branch vein occlusion. The affected segment of retina shows changes of reduced perfusion. This results in irregularity of the arterioles and veins, areas of capillary closure, and dilated capillaries with microaneurysms.
The value of laser treatment in the management of the complications of branch retinal vein occlusion is discussed in Chapters 10 and 24.
ATHEROSCLEROSIS & ARTERIOSCLEROSIS
The process of atherosclerosis occurs in larger arteries and is due to fatty infiltration of a patchy nature occurring in the intima and associated with fibrosis. Involvement of smaller vessels (ie, < 300 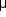 m) by diffuse fibrosis and hyalinization is termed arteriosclerosis. The retinal vessels beyond the disk are less than 300
m) by diffuse fibrosis and hyalinization is termed arteriosclerosis. The retinal vessels beyond the disk are less than 300  m; therefore, involvement of the retinal arterioles should be termed arteriosclerosis, whereas involvement of the central retinal artery is properly termed atherosclerosis.
m; therefore, involvement of the retinal arterioles should be termed arteriosclerosis, whereas involvement of the central retinal artery is properly termed atherosclerosis.
Atherosclerosis is a progressive change developing in the second decade, with lipid streaks in larger vessels, progressing to a fibrous plaque in the third decade. In the fourth and fifth decades, ulceration, hemorrhages, and thrombosis occur, and the lesion may be calcified. Destruction of the elastic and muscular elements of the media produces ectasia and rupture of the large vessels, though in smaller vessels obstruction is usually seen. The clinical results of atherosclerosis are seen several decades after the onset of the process. Factors contributing to atheroma include hyperlipidemia, hypertension, and obesity.
Arteriosclerosis is characterized by an enhanced light reflection, focal attenuation, and irregularity of caliber. These signs may also be seen in the arterioles of normotensive individuals in middle age. In elderly individuals with arteriosclerosis and associated mild hypertension, it is difficult to differentiate the changes of arteriosclerosis from those due to hypertension.
Appearance of Retinal Vessels
A normal arteriolar wall is transparent, so that what is actually seen is the column of blood within the vessel. A thin, central light reflection in the center of the blood column appears as a yellow refractile line about one-fifth the width of the column. As the walls of the arterioles become infiltrated with lipids and cholesterol, the vessels become sclerotic. As this process continues, the vessel wall gradually loses its transparency and becomes visible; the blood column appears wider than normal, and the thin light reflection becomes broader. The grayish yellow fat products in the vessel wall blend with the red of the blood column to produce a typical "copper wire" appearance. This indicates moderate arteriosclerosis. As sclerosis proceeds, the blood column-vessel wall light reflection resembles "silver wire," which indicates severe arteriosclerosis; at times, even occlusion of an arteriolar branch may occur.
Red-free light (a white light with a green filter) allows details of hemorrhages, focal irregularity of blood vessels, and nerve fibers to be seen more clearly (Figure 15-9).
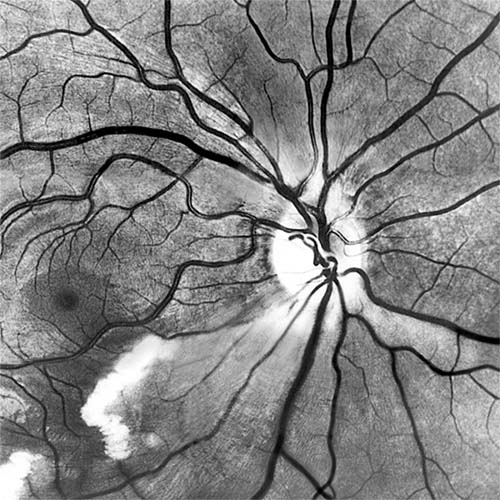
Figure 15-9: Acute retinal infarction. Red-free photograph shows acute arterial occlusion in a congenitally anomalous vessel at the disk. The inferior retina is infarcted, but axoplasm has accumulated beneath the fovea in an irregular pattern owing to preserved neuronal function of the distal ganglion cells.
HYPERTENSIVE RETINOPATHY
Wagener and Keith in 1939 classified patients with hypertensive retinopathy into four groups. Stages I and II were restricted to arteriolar changes with attenuation and an increased light reflection ("copper" or "silver" wiring). More emphasis has been placed on stages III and IV, which include cotton-wool spots, hard exudates, hemorrhages, and extensive microvascular changes. Stage IV is differentiated by the additional feature of edema of the optic disk.
The appearance of the fundus in hypertensive retinopathy is determined by the degree of elevation of the blood pressure and the state of the retinal arterioles. Thus, in young patients with accelerated hypertension, an extensive retinopathy is seen, with hemorrhages, retinal infarcts (cotton-wool spots), choroidal infarcts (Elschnig's spots), and occasionally serous detachment of the retina (Figure 15-10). Severe disk edema is a prominent feature. Vision may be impaired but is restored if blood pressure is reduced with caution.
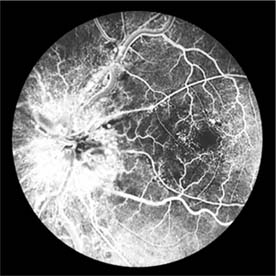
Figure 15-10: Accelerated hypertension. Fluorescein angiogram in a young man showing arteriolar constriction, dilation of capillaries with microaneurysms, and areas of closure. Marked disk edema is present.
In contrast, elderly patients with arteriosclerotic vessels are unable to respond in this manner, and their vessels are thus protected by the arteriosclerosis. It is for this reason that elderly patients seldom exhibit florid hypertensive retinopathy (Figure 15-11).
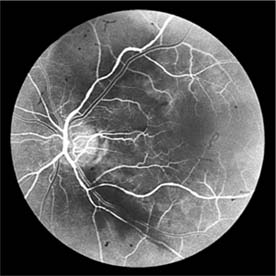
Figure 15-11: Accelerated hypertension. Fluorescein angiogram in an elderly wom.an showing marked arteriolar constriction and irregularity but few signs of florid retinopathy.
Fluorescein angiography has made possible accurate documentation of these microcirculatory changes. In young patients with hypertension, arteriolar attenuation and occlusion are seen, and capillary nonperfusion can be verified in relation to a cotton-wool spot, which is surrounded by abnormal dilated capillaries and microaneurysms with increased permeability on fluorescein angiography.
Resolution of the cotton-wool spots and the arteriolar changes occurs with successful hypotensive therapy. In elderly patients, the underlying arteriosclerotic changes are irreversible.
Other Forms of Hypertensive Retinopathy
A severe retinopathy may be seen in advanced renal disease, in patients with pheochromocytoma, and in preeclampsia-eclampsia. All such patients should receive a complete medical workup to establish the nature of the hypertension.
CHRONIC OCULAR ISCHEMIA
Reduction in the retinal arteriovenous pressure gradient may produce acute signs of ocular ischemia (see preceding pages) or the less frequently recognized chronic changes.
Carotid Occlusive Disease
Carotid occlusive disease usually presents in middle-aged and elderly patients and is due to involvement of both the carotid artery and its smaller branches. Contributory factors include hypertension, smoking, and hyperlipidemia.
In anterior segment ischemia, patients develop iritis, intraocular pressure changes, and pupillary abnormalities. In retinal ischemia (Figure 15-12A), patients show evidence of capillary dilation and hemorrhages, capillary occlusion, new vessels at the optic disk, and cotton-wool spots.
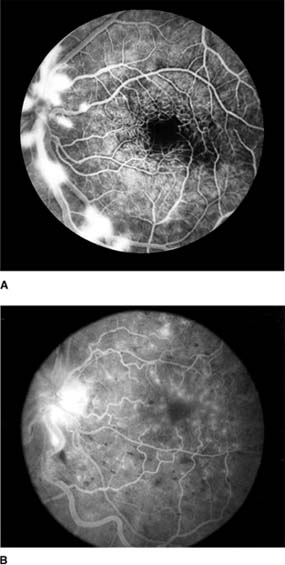
Figure 15-12: A: Fluorescein angiogram of left fundus in a patient with chronic ocular ischemia secondary to Takayasu's disease. Note capillary dilation, leakage of dye, retinal hemorrhages, cotton-wool spots, and neovascularization of the optic nerve head. B: Fluorescein angiogram, showing leakage at optic disk and macula in a patient with chronic ocular ischemia secondary to dural arteriovenous fistula.
Carotid Cavernous Fistula
Carotid cavernous fistula results from a communication between the carotid artery or its branches and the cavernous sinus, producing characteristic vascular signs. Direct carotid fistulas are usually acute, florid, and posttraumatic, whereas fistulas from dural vessels are usually chronic, mild, and not associated with trauma. Clinical features include elevated intraocular pressure, dilated conjunctival vessels, dilated retinal vessels with hemorrhages and fluorescein leakage (Figure 15-12B), ophthalmoplegia (usually lateral rectus), and bruit. computed tomography (CT) and magnetic resonance imaging (MRI) show thickened ocular muscles and a dilated superior ophthalmic vein. The condition must be differentiated from thyroid eye disease, and interventional radiology is the ultimate diagnostic and therapeutic resource.
IDIOPATHIC (BENIGN) INTRACRANIAL HYPERTENSION (Pseudotumor Cerebri)
Idiopathic intracranial hypertension is raised intracranial pressure without other cerebrospinal fluid abnormalities and with normal radiologic studies. Patients present with headache, tinnitus, and dizziness; blurred vision, and diplopia are the ophthalmologic features. Etiologic factors include (1) drug therapy, particularly oral contraceptives, nalidixic acid, tetracyclines, sulfonamides, vitamin A, and prolonged steroid therapy or steroid withdrawal in children; (2) endocrine abnormalities; and (3) blood dyscrasias. In many cases there is no obvious cause; in this (idiopathic) group, the patients are usually young overweight women with irregular menstrual cycles. Idiopathic intracranial hypertension is very rare in men.
The cause of the increased intracranial pressure is unknown, though diminished absorption of cerebrospinal fluid due to impaired venous sinus drainage is suspected.
On examination, visual fields are initially normal apart from enlarged blind spots due to papilledema. Generalized field constriction and inferonasal and arcuate defects occur in advanced cases. Cerebrospinal fluid pressure is raised. MRI shows distended nerve sheaths, an empty sella, and absence of a mass lesion. MR angiography complements the examination and detects any venous sinus occlusion. The aims of treatment are to reduce spinal fluid pressure and prevent permanent visual loss associated with optic atrophy, which occurs in up to 50% of patients. Treatment includes strict diet, oral acetazolamide, optic nerve sheath decompression, and lumboperitoneal shunt procedures. Optic nerve sheath decompression functions either as a fistula or by producing subarachnoid fibrous tissue in the nerve sheath and thus protects the disk from the raised sheath pressure. This procedure is relatively free of side effects and complications.
SUBACUTE INFECTIVE ENDOCARDITIS
Inflammatory changes on the cardiac valves may produce multiple embolization with frequent ocular manifestations that range from retinal and choroidal infarction to a mild infective vitritis. The emboli may arise from vegetations on the cardiac valves and may be composed of platelet and fibrinogen aggregates or calcified endocardial vegetations (Figure 15-13).
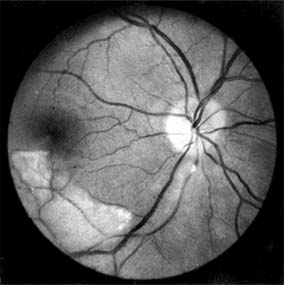
Figure 15-13: Subacute bacterial endocarditis. Calcific embolus impacted in arteriole below the disk, producing a distal area of retinal infarction.
Page 1 of 17
10.1036/1535-8860.ch15