Chapter 11: Glaucoma
PRIMARY GLAUCOMA
PRIMARY OPEN-ANGLE GLAUCOMA
Primary open-angle glaucoma is the most common form. About 0.4-0.7% of persons over age 40 and 2-3% of persons over age 70 are estimated to have primary open-angle glaucoma. The disease is four times more common and generally more aggressive in blacks. There is a strong familial tendency in primary open-angle glaucoma, and close relatives of affected individuals should undergo regular screening.
The chief pathologic feature of primary open-angle glaucoma is a degenerative process in the trabecular meshwork, including deposition of extracellular mate-rial within the meshwork and beneath the endothelial lining of Schlemm's canal. This differs from the normal aging process. The consequence is a reduction in aqueous drainage leading to a rise in intraocular pressure.
Juvenile-onset open-angle glaucoma (a familial primary open-angle glaucoma with early onset), about 5% of familial cases of primary open-angle glaucoma, and about 3% of nonfamilial cases of primary open-angle glaucoma are associated with mutations in a gene on chromosome 1. This gene causes trabecular meshwork cells to produce an extracellular protein known as trabecular meshwork-inducible glucocorticoid response (TIGR) as a result of its association with glaucoma secondary to steroid therapy. It is suggested that these mutations result in abnormal amounts or types of TIGR. A mutation on chromosome 3 has also been implicated in autosomal dominant adult-onset primary open-angle glaucoma.
Raised intraocular pressure precedes optic disk and visual field changes by months to years. Although there is a clear association between the level of intraocular pressure and the severity and rate of progression of visual loss, there is great variability between individuals in the effect on the optic nerve of a given pressure elevation. Some people tolerate elevated intraocular pressure without developing disk or field changes (ocular hypertension; see below); others develop glaucomatous changes with consistently "normal" intraocular pressure (low-pressure glaucoma; see below).
The mechanism of neuronal damage in primary open-angle glaucoma and its relationship to the level of intraocular pressure is much debated. The major theories implicate intraocular pressure-dependent changes (as discussed above) or reduction in the vascular supply to the optic nerve head.
Higher levels of intraocular pressure are associated with greater field loss at presentation. When there is glaucomatous field loss on first examination, the risk of further progression is much greater. Since intraocular pressure is the only treatable risk factor, it remains the focus of therapy. There is strong evidence that control of intraocular pressure slows disk damage and field loss.
In the patient with extensive disk changes or field loss, it is advisable to reduce the intraocular pressure as much as possible, whereas a patient with only a suspicion of disk or field changes may need less vigorous treatment. In all cases, the inconveniences and possible complications of treatment must be considered. Many glaucoma patients are old and frail and may not tolerate vigorous treatment. In order to gain a perspective on the need for treatment, an initial period of observation without treatment may be necessary to determine the rate of progression of disk and field changes. There is no justification for subjecting an elderly patient to extremes of treatment when the likelihood of their developing significant visual loss during their lifetime is small.
Diagnosis
The diagnosis of primary open-angle glaucoma is established when glaucomatous optic disk or field changes are associated with elevated intraocular pressures, a normal-appearing open anterior chamber angle, and no other reason for intraocular pressure elevation. Approximately 50% of patients with primary open-angle glaucoma have a normal intraocular pressure when first examined, so repeated tonometry is necessary before the diagnosis can be established.
Screening for Glaucoma
The major problem in detection of primary open-angle glaucoma is the absence of symptoms until relatively late in the disease. When patients first notice field loss, substantial glaucomatous cupping has already occurred. If treatment is to be successful, it must be started early in the disease, and this depends upon an active screening program. Unfortunately, glaucoma screening programs are hampered by the unreliability of a single intraocular pressure measurement in the detection of primary open-angle glaucoma and the complexities of relying on optic disk or visual field changes. At present it is necessary to rely for early diagnosis on regular ophthalmologic assessment of first-degree relatives of affected individuals.
Medical Treatment of Glaucoma
A. Suppression of Aqueous Production:
Topical beta-adrenergic blocking agents are now the most widely used form of glaucoma therapy. They may be used alone or in combination with other drugs. Timolol maleate 0.25% and 0.5%, betaxolol 0.25% and 0.5%, levobunolol 0.25% and 0.5%, metipranolol 0.3%, and carteolol 1% are the currently available preparations. The major contraindications to their use are chronic obstructive airways disease-particularly asthma-and cardiac conduction defects. Betaxolol, with its relatively greater selectivity for 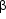 1 receptors, less often produces respiratory side effects but is also less effective at reducing intraocular pressure. Depression, confusion, and fatigue may occur with the topical beta-blocking agents.
1 receptors, less often produces respiratory side effects but is also less effective at reducing intraocular pressure. Depression, confusion, and fatigue may occur with the topical beta-blocking agents.
Apraclonidine is an  2-adrenergic agonist that decreases aqueous humor formation without effect on outflow. Epinephrine and dipivefrin have some effect on aqueous production (see below).
2-adrenergic agonist that decreases aqueous humor formation without effect on outflow. Epinephrine and dipivefrin have some effect on aqueous production (see below).
Brimonidine 0.2% is a new alpha-adrenergic agonist that primarily inhibits aqueous production and secondarily increases aqueous outflow. It shows promise both as a first-line antiglaucoma drug and as an adjunctive agent.
Dorzolamide hydrochloride 2% is a recently developed topical carbonic anhydrase inhibitor that is especially effective when employed adjunctively, though not as effective as systemic carbonic anhydrase inhibitors. Combining dorzolamide and timolol in the same solution is under investigation.
Systemic carbonic anhydrase inhibitors-acetazolamide is the most widely used, but dichlorphenamide and methazolamide are alternatives-are used in chronic glaucoma when topical therapy is insufficient and in acute glaucoma when very high intraocular pressure needs to be controlled quickly. They are capable of suppressing aqueous production by 40-60%. Acetazolamide can be administered orally in a dosage of 125-250 mg up to four times daily or as Diamox Sequels 500 mg once or twice daily, or it can be given intravenously (500 mg). The carbonic anhydrase inhibitors are associated with major systemic side effects that limit their usefulness for long-term therapy.
Hyperosmotic agents influence aqueous production as well as dehydrating the vitreous body (see below).
B. Facilitation of Aqueous Outflow:
Parasympathomimetic agents increase aqueous outflow by action on the trabecular meshwork through contraction of the ciliary muscle. Pilocarpine is the most commonly used drug in this group. It is given as 0.5-6% solution instilled several times a day or as 4% gel instilled at bedtime. Carbachol 0.75-3% is an alternative cholinergic agent. Irreversible anticholinesterase agents are the longest-acting parasympathomimetics available. These include demecarium bromide, 0.125% and 0.25%, and echothiophate iodide, 0.03-0.25%, which are generally restricted to aphakic or pseudophakic patients because of their cataractogenic potential. Caution: The irreversible anticholinesterase agents will potentiate succinylcholine administered during anesthesia, and anesthetists must be appropriately warned prior to surgery.
All parasympathomimetic agents produce miosis with dimness of vision, particularly in patients with cataract, and accommodative spasm that may be disabling to younger patients. Retinal detachment is a serious but rare occurrence.
Latanoprost 0.005%, a prostaglandin F2 analog, acts as an antiglaucoma drug by increasing the uveoscleral outflow of aqueous. It is used once daily in the evening and may work alone or as an adjunct to other antiglaucoma agents. To date there have been no systemic side effects, and once-daily dosing is a distinct advantage. However, it does increase iris pigmentation.
analog, acts as an antiglaucoma drug by increasing the uveoscleral outflow of aqueous. It is used once daily in the evening and may work alone or as an adjunct to other antiglaucoma agents. To date there have been no systemic side effects, and once-daily dosing is a distinct advantage. However, it does increase iris pigmentation.
Epinephrine, 0.25-2% instilled once or twice daily, increases aqueous outflow with some decrease in aqueous production. There are a number of external ocular side effects, including reflex conjunctival vasodilation, adrenochrome deposits, follicular conjunctivitis, and allergic reactions. Dipivefrin is a prodrug of epinephrine that is metabolized intraocularly to its active state. Neither epinephrine nor dipivefrin should be used in eyes with narrow anterior chamber angles.
C. Reduction of Vitreous Volume:
Hyperosmotic agents render the blood hypertonic, thus drawing water out of the vitreous and causing it to shrink. This is in addition to decreasing aqueous production. Reduction in vitreous volume is helpful in the treatment of acute angle-closure glaucoma and in malignant glaucoma when anterior displacement of the crystalline lens (caused by volume changes in the vitreous or choroid) produces angle closure (secondary angle-closure glaucoma).
Oral glycerin (glycerol), 1 mL/kg of body weight in a cold 50% solution mixed with lemon juice, is the most commonly used agent, but it should be used with care in diabetics. Alternatives are oral isosorbide and intravenous urea or mannitol (see Chapter 3 for dosages).
D. Miotics, Mydriatics, and Cycloplegics:
Constriction of the pupil is fundamental to the management of primary angle-closure glaucoma and the angle crowding of plateau iris. Pupillary dilation is important in the treatment of angle closure secondary to iris bombé due to posterior synechiae.
When angle closure is secondary to anterior lens displacement, cycloplegics (cyclopentolate and atropine) are used to relax the ciliary muscle and thus tighten the zonular apparatus in an attempt to draw the lens backward.
Surgical & Laser Treatment of Glaucoma
A. Peripheral Iridotomy and Iridectomy:
Pupillary block in angle-closure glaucoma is most satisfactorily overcome by forming a direct communication between the anterior and posterior chambers that removes the pressure difference between them. This is best done with the neodymium: YAG laser. Surgical peripheral iridectomy is performed if laser iridotomy is ineffective. YAG laser iridotomy is preventive when used in patients with narrow angles before closure attacks occur
B. Laser Trabeculoplasty:
Application of laser (usually argon) burns via a goniolens to the trabecular meshwork facilitates aqueous outflow by virtue of its effects on the trabecular meshwork and Schlemm's canal or cellular events that enhance the function of the meshwork. The technique is applicable to many forms of open-angle glaucoma, and the results are variable depending upon the underlying cause. The pressure reduction usually allows decrease of medical therapy and postponement of glaucoma surgery. Treatments can be repeated (see Chapter 24). Laser trabeculoplasty may be used in the initial treatment of primary open-angle glaucoma. In most cases, the intraocular pressure gradually returns to the pretreatment level 2-5 years later
C. Glaucoma Drainage Surgery:
The increased effectiveness of medical and laser treatment has reduced the need for glaucoma drainage surgery, but surgery is able to produce a more marked reduction in intraocular pressure.
Trabeculectomy is the procedure most commonly used to bypass the normal drainage channels, allowing direct access from the anterior chamber to the subconjunctival and orbital tissues. The major complication is fibrosis in the episcleral tissues, leading to closure of the new drainage pathway. This is most likely to occur in young patients, blacks, in patients with glaucoma secondary to uveitis, and in those who have previously undergone glaucoma drainage surgery or other surgery involving the episcleral tissues. Adjunctive treatment with antimetabolites such as fluorouracil and mitomycin reduces the risk of bleb failure but may lead to other bleb-related complications or maculopathy from persistent ocular hypotony.
Implantation of a silicone tube to form a permanent conduit for aqueous flow out of the eye is an alternative procedure for eyes that are unlikely to respond to trabeculectomy. This includes eyes with secondary glaucoma, particularly neovascular glaucoma, and glaucoma following corneal graft surgery.
Goniotomy is a useful technique in treating primary congenital glaucoma, in which there appears to be an obstruction to aqueous drainage in the internal portion of the trabecular meshwork.
D. Cyclodestructive Procedures:
Failure of medical and surgical treatment in advanced glaucoma may lead to consideration of laser or surgical destruction of the ciliary body to control intraocular pressure. Cryotherapy, diathermy, high-frequency ultrasound, and thermal mode neodymium:YAG laser therapy can all be used to cause destruction of the ciliary body.
Course & Prognosis
Without treatment, open-angle glaucoma may be insidiously progressive to complete blindness. If antiglaucoma drops control the intraocular pressure in an eye that has not suffered extensive glaucomatous damage, the prognosis is good (though visual field loss may progress in spite of normalized intraocular pressure). When the process is detected early, most glaucoma patients can be successfully managed medically.
NORMAL-PRESSURE GLAUCOMA (Low-Pressure Glaucoma)
A minority of patients with glaucomatous optic disk or visual field changes have an intraocular pressure consistently below 25 mm Hg. These patients have normal- or low-pressure glaucoma. The pathogenesis involves an abnormal sensitivity to intraocular pressure because of vascular or mechanical abnormalities at the optic nerve head. Disk hemorrhages are more frequently seen in normal-pressure than in primary open-angle glaucoma and often herald progression of field loss.
Before the diagnosis of low-pressure glaucoma can be established, a number of entities must be excluded:
Prior episode of raised intraocular pressure, such as caused by iridocyclitis, trauma, or topical steroid therapy.
Large diurnal variation in intraocular pressure with significant elevations, usually early in the morning.
Postural changes in intraocular pressure with a marked elevation when lying flat.
Intermittent elevations of intraocular pressure such as in subacute angle closure.
Other causes of optic disk and field changes, including congenital disk abnormalities and acquired optic atrophy due to tumors or vascular disease.
OCULAR HYPERTENSION
Ocular hypertension is elevated intraocular pressure without disk or field abnormalities and is more common than primary open-angle glaucoma. The rate at which such individuals develop glaucoma is approximately 5-10 per 1000 per year. The risk increases with increasing intraocular pressure, increasing age, a positive family history for glaucoma, myopia, diabetes mellitus, and cardiovascular disease. It is also increased in blacks. The development of disk hemorrhages in a patient with ocular hypertension also indicates an increased risk for development of glaucoma.
Patients with ocular hypertension are considered glaucoma suspects and should undergo regular monitoring (one to three times a year) of the optic disk, intraocular pressure, and visual fields.
PRIMARY ACUTE ANGLE-CLOSURE GLAUCOMA
Primary acute angle-closure glaucoma occurs when sufficient iris bombé develops to cause occlusion of the anterior chamber angle by the peripheral iris. This blocks aqueous outflow and the intraocular pressure rises rapidly, causing severe pain, redness, and blurring of vision. Angle-closure glaucoma occurs in eyes with preexisting anatomic narrowing of the anterior chamber angle (found mainly in hyperopes). The acute attack generally occurs in older patients when there has been enlargement of the crystalline lens associated with aging. In angle-closure glaucoma, the pupil is mid-dilated, with associated pupillary block. This usually occurs in the evenings, when the level of illumination is reduced. It may also occur with pupillary dilation for ophthalmoscopy. If pupillary dilation is necessary in a patient with a shallow anterior chamber (easily detected by oblique illumination with a penlight [Figure 11-4] and then confirmed by gonioscopy), it is best to rely on short-acting mydriatics and observe the patient carefully.
Clinical Findings
Acute angle-closure glaucoma is characterized by a sudden onset of severe blurring followed by excruciating pain, halos, and nausea and vomiting. Other findings include markedly increased intraocular pressure, a shallow anterior chamber, a steamy cornea, a fixed, moderately dilated pupil, and ciliary injection. It is important to perform gonioscopy on the fellow eye.
Differential Diagnosis
Acute iritis causes more photophobia than acute glaucoma. Intraocular pressure is usually not elevated; the pupil is constricted; and the cornea is usually not edematous. Marked flare and cells are present in the anterior chamber, and there is deep ciliary injection.
In acute conjunctivitis, there is little or no pain and no visual loss. There is discharge from the eye and an intensely inflamed conjunctiva but no ciliary injection. The pupillary responses and intraocular pressure are normal, and the cornea is clear.
Complications & Sequelae
If treatment is delayed, the peripheral iris may adhere to the trabecular meshwork (anterior synechiae), producing irreversible occlusion of the anterior chamber angle requiring surgery. Optic nerve damage is common.
Treatment
Acute angle-closure glaucoma is an ophthalmic emergency!
Treatment is initially directed at reducing the intraocular pressure. Intravenous and oral acetazol-amide-along with hyperosmotic agents and topical beta-blockers-will usually reduce the intraocular pressure. Pilocarpine 4% can then be used intensively, eg, 1 drop every 15 minutes for 1 hour. Epinephrine must not be used because it will accentuate angle closure.
Once the intraocular pressure is under control, peripheral iridectomy should be undertaken to form a permanent connection between the anterior and posterior chambers, thus preventing recurrence of iris bombé. This is most often done with the neo-dymium:YAG laser. Surgical peripheral iridectomy is indicated if laser treatment is unsuccessful.
In most cases, the fellow eye should undergo prophylactic laser iridotomy.
SUBACUTE ANGLE-CLOSURE GLAUCOMA
The same etiologic factors operate in subacute as in acute angle-closure glaucoma except that episodes of elevated intraocular pressure are of short duration and are recurrent. The episodes of angle closure resolve spontaneously, but there is accumulated damage to the anterior chamber angle, with formation of peripheral anterior synechiae. Subacute angle closure will occasionally progress to acute closure.
There are recurrent short episodes of unilateral pain, redness, and blurring of vision associated with halos around lights. Attacks often occur in the evenings and resolve overnight. Examination between attacks may show only a narrow anterior chamber angle. The diagnosis can be confirmed by gonioscopy.
Treatment is similar to that of primary angle- closure glaucoma.
CHRONIC ANGLE-CLOSURE GLAUCOMA
A small number of patients with the predisposition to a anterior chamber angle closure never develop episodes of acute rise in intraocular pressure but form increasingly extensive peripheral anterior synechiae accompanied by a gradual rise in intraocular pressure. These patients present in the same way as those with primary open-angle glaucoma, often with extensive visual field loss in both eyes. Occasionally, they have attacks of subacute angle closure.
On examination, there is elevated intraocular pressure, narrow anterior chamber angles with variable amounts of peripheral anterior synechiae, and optic disk and visual field changes.
Once again, peripheral iridectomy is an important component of treatment. (Laser iridotomy in these patients is liable to produce a marked rise in intraocular pressure.) Intraocular pressure is then controlled medically if possible, but the extent of peripheral anterior synechia formation and sluggish outflow through the remaining trabecular meshwork make pressure control very difficult, so that drainage surgery is often required. Epinephrine and strong miotics must not be used unless peripheral iridectomy has been performed because they will accentuate angle closure.
PLATEAU IRIS
Plateau iris is an uncommon condition in which the central anterior chamber depth is normal but the anterior chamber angle is very narrow owing to a congenital high insertion of the iris. Such an eye has little pupillary block, but dilation will cause bunching up of the peripheral iris, occluding the angle (angle crowding) even if peripheral iridectomy has been performed. Affected individuals present with acute angle-closure glaucoma at a young age, with recurrences after peripheral iridectomy. Long-term miotic therapy or laser iridoplasty is required.
Pupillary dilation for fundus examination is apt to cause acute angle closure in patients with plateau iris and may precipitate a similar event in other eyes with deep anterior chambers-due to angle crowding rather than the pupillary block mechanism seen in eyes with shallow anterior chambers.
PREVIOUS | NEXT
Page: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
10.1036/1535-8860.ch11