
|
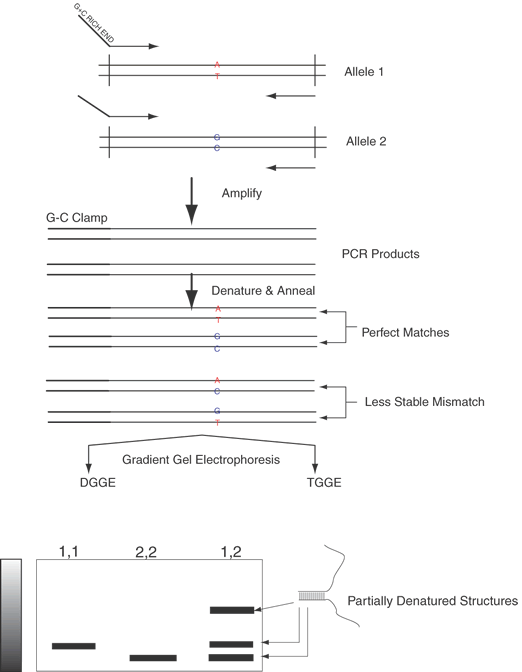
| Fig. 13. Denaturing gradient gel electrophoresis (DGGE) and thermal gradient gel electrophoresis (TGGE). Another approach to analysis and detection of single-base changes is the exploitation of the difference in melting behavior resulting from a single base change. PCR-amplified products are used as before except that one primer contains an extra sequence at the 5' end, which is G + C rich. This sequence when amplified is called a G-C clamp, and it melts at very high temperature in comparison to the typical sequence adjacent to it. In DGGE, the typical sequences will melt at varying denaturant concentrations along the solvent gradient. When they melt, the structure becomes Y-shaped because of the very stable G-C clamp. The resulting Y-shaped structure migrates extremely slowly in the gel. The gel contains a linear gradient of a chemical denaturant, urea, more concentrated at the bottom than the top. Thus, a double-stranded DNA migrates at its normal rate in a gel until it encounters a urea concentration high enough to partially denature the double-stranded DNA into the Y-shaped structure, and then it appears to stop. Different DNA sequences will migrate to different points along the urea gradient before converting to the Y-shaped structure. If two sequences that differ only by one base are run side by side, it is difficult to predict which will stop migrating sooner, but we might expect that one with an A : T base-pair would melt before an identical sequence excepting for a G:C base pair. However, heteroduplexes (double-stranded sequences containing one or more mismatches) with even a single mismatch of an A with a C or a G with a T would be expected to form Y-shaped structures at considerably lower concentrations of denaturant than strands that are perfectly matched and that bear the same flanking sequences. Similarly, in TGGE there is a temperature gradient within the gel increasing from cathode to anode; that is, the gel is hotter at the bottom than the top. The temperature gradient replaces the chemical denaturant gradient required in DGGE. The heteroduplexes melt at a lower temperature than the homoduplexes, at about 1.5°C per 1% difference; thus, a single-base change results in a significant change in the denaturation point. Following PCR assay, a heterozygote (shown here as an A to G substitution, in otherwise identical sequences) sample will contain four different pairs of sequences: two homoduplexes (1:1 and 2:2), and two heteroduplexes (1:2 and 2:1). Homozygous individuals (1,1 and 2,2) are shown for comparison. Their predicated mobilities on DGGE and TGGE are indicated. |