
|
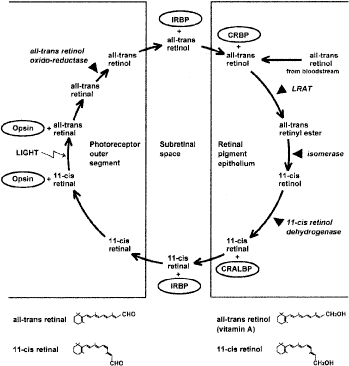
| Fig. 8. Schematic diagram of the mammalian retinoid cycle in vision and the structure of the principal retinoids. Starting at the one o'clock position in the figure, the retinal pigment epithelium receives all-trans retinol (vitamin A) from the bloodstream or from photoreceptor cells when light bleaches cone or rod photopigments. In the retinal pigment epithelium, all-trans retinol is complexed with cellular retinol-binding protein (CRBP). Retinol is esterified to fatty acids, such as palmitic or stearic acid, by the enzyme lecithin:retinol acyltransferase (LRAT). The retinyl esters are the substrate for isomerohydrolase (isomerase), which produces 11-cis retinol. The diagram shows 11-cis retinol being oxidized to 11-cis retinal by the enzyme 11-cis retinol dehydrogenase. However, at this point there is an alternative pathway not shown in the diagram for simplicity, whereby 11-cis retinol is esterified and stored; the 11-cis retinyl esters are ultimately hydrolyzed to recreate 11-cis retinol, which is the substrate for 11-cis retinol dehydrogenase. In the retinal pigment epithelium, 11-cis retinal is complexed with cellular retinaldehyde-binding protein (CRALBP), whereas in the subretinal space it is complexed with interphotoreceptor retinoid binding protein (IRBP). On entering the cone and rod photoreceptors, 11-cis retinal functions as the chromophore in the cone opsins or rod opsin (rhodopsin). After exposure to light, the chromophore is converted to all-trans retinal. All-trans retinal dissociates from the opsins and, before leaving the photoreceptor cells, is reduced to all-trans retinol by all-trans retinol oxido-reductase. (Courtesy of Drs H Yamamoto, A Simon, U Eriksson, E Harris, EL Berson, and TP Dryja.) |